Figure 2.
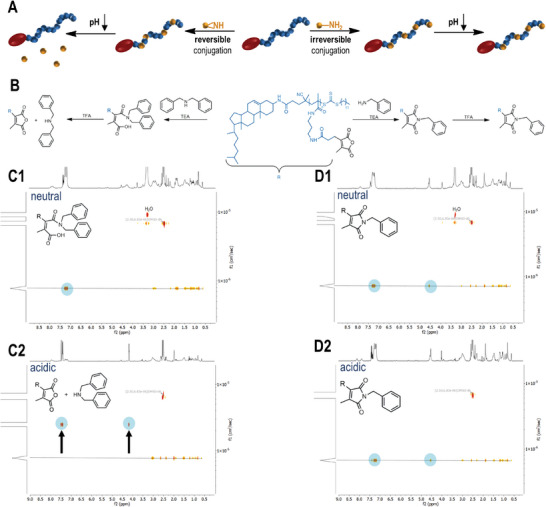
pH sensitivity of chol‐p(PMMA‐MA)30 conjugated with different amines. A) Scheme for the conversion of chol‐polymeric 2‐porpionic‐3‐methylmaleic anhydride groups with primary or secondary amines and their corresponding pH response. B) Amidation of 2‐propionic‐3‐methylmaleic anhydride side groups with benzyl‐ or dibenzylamine affording a pH‐reversible or irreversible polymer system. C1) 1H DOSY NMR spectrum of the chol‐polymer treated with dibenzylamine providing an identical diffusion species under neutral conditions and C2) the related 1H DOSY NMR spectrum upon acidification showing the successful release of the aromatic compound. D1) 1H DOSY NMR spectrum of the chol‐polymer treated with benzylamine under neutral and D2) acidic conditions affording only one diffusion species and no release of the primary amine upon acidification.
