Figure 4.
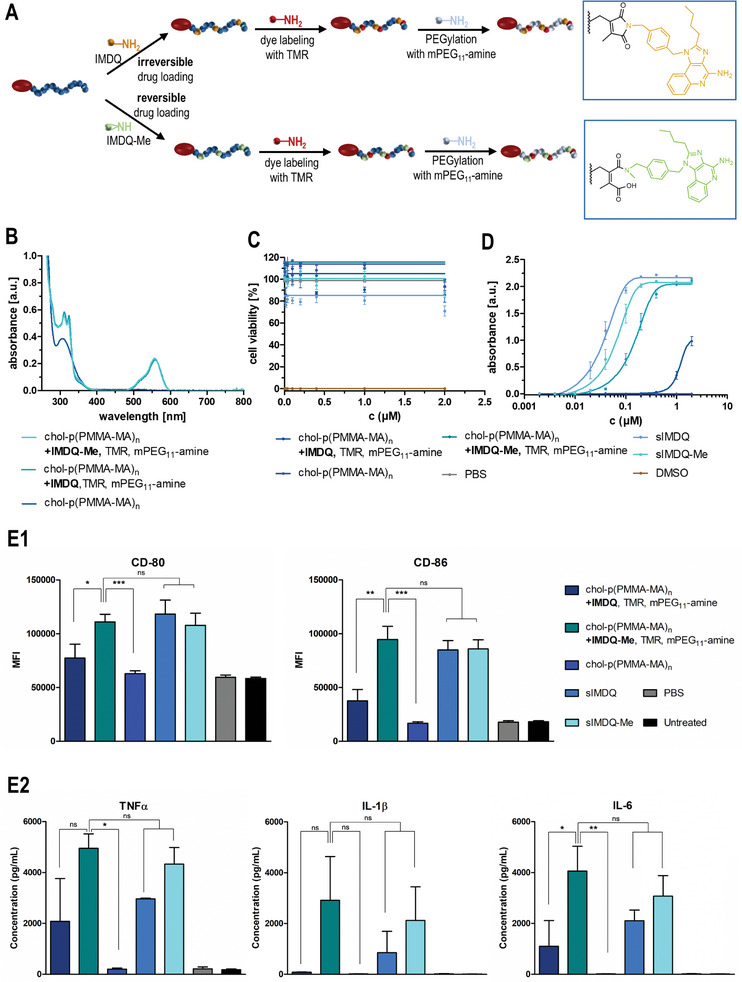
In vitro characterization of drug‐loaded cholesteryl‐linked polymers with 2‐propionic‐3‐methylmaleic anhydride side groups. A) Synthetic strategy for the formulation of a reversibly (IMDQ‐Me) or irreversibly (IMDQ)‐loaded and TMR‐labeled chol‐p(PMMA‐MA)32 hydrophilized with PEG11‐amine. B) UV–vis spectra of the corresponding drug‐loaded and dye‐labeled polymer systems as well as the unmodified chol‐polymer. C) Cell viability assay (MTT) of RAW‐Dual macrophages incubated with the soluble drugs (IMDQ and IMDQ‐Me) and respective drug‐loaded chol‐polymers, the unmodified chol‐polymer as well as PBS (positive control) and DMSO (negative control) (n = 4). D) TLR receptor activation of RAW‐Dual macrophages incubated with the drug‐conjugated chol‐polymers, free drugs, or empty chol‐polymer quantified by RAW blue assay (n = 4). E) Maturation of bone marrow‐derived dendritic cells after incubation with soluble drugs (IMDQ and IMDQ‐Me) and respective drug‐loaded chol‐polymers, the unmodified chol‐polymer as well as PBS. E1) To delineate the cellular activation state, the expression of the costimulatory markers CD80 and CD86 was quantified via flow cytometry by mean fluorescence intensity (MFI) (n = 3, *: p ≤ 0.1, **: p ≤ 0.05, +: p ≤ 0.01). E2) Additionally, the secretion of the proinflammatory cytokines TNFα, IL 1β, and IL‐6 into the cell culture media was quantified by cytometric bead assay (n = 3, *: p ≤ 0.1, **: p ≤ 0.05, ***: p ≤ 0.01).
