Figure 5.
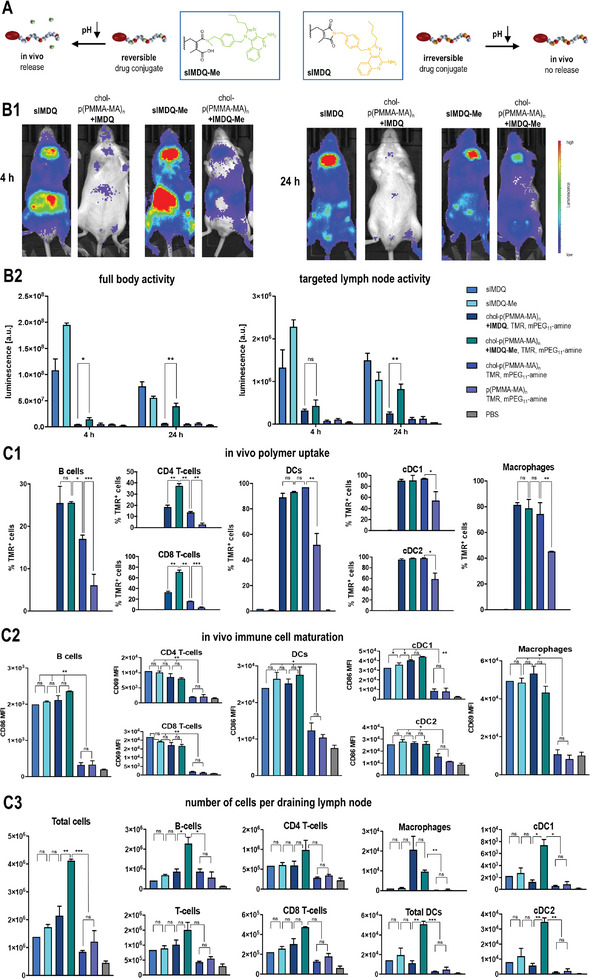
In vivo performance of chol‐polymer with pendant 2‐propionic‐3‐methylmaleic anhydride groups covalently attached with the TLR 7/8 agonist IMDQ or methylated IMDQ‐Me. A) Scheme for the exclusive acid‐triggered drug release for IMDQ‐Me compared to IMDQ, mediated by the dye‐ and drug‐loaded chol‐p(PMMA‐MA)32 polymer, further PEGylated with mPEG11‐amine. B) Immune stimulatory properties of those samples after footpad injection into BALB/c IFN‐β (IFN‐β +/Δ β ‐luc) luciferase reporter. B1) Representative luminescence images of the respective samples after 4 and 24 h and B2) corresponding luminescence quantification of full‐body activity and targeted lymph node activity, which is only fully restored for the mice treated with IMDQ‐Me‐loaded chol‐p(PMMA‐MA)32 (n = 3 or 4, *: p ≤ 0.1, **: p ≤ 0.05, ***: p ≤ 0.01). C) Flow cytometric analyses of the draining popliteal lymph nodes 24 h after footpad injection (n = 2 or 3): C1) The uptake of the (chol)‐p(PMMA‐MA)32 polymer was followed by TMR fluorescence and confirmed effective cholesteryl end group mediated delivery, C2) the maturation of the respective immune cell subpopulations was evaluated by mean fluorescence intensity of the respective maturation markers CD86 or CD69 and confirmed the immunostimulatory activity of the TLR 7/8 agonists, C3) the number of cells in the lymph node was determined in relation to count beads and revealed effective immunodrug delivery properties for chol‐p(PMMA‐MA)n carrying the pH‐releasable TLR 7/8 agonist IMDQ‐Me (n = 3, *: p ≤ 0.1, **: p ≤ 0.05, ***: p ≤ 0.01).
