Abstract
Acid sphingomyelinase deficiency (ASMD) is an ultra‐rare lysosomal storage disease with a broad spectrum of manifestations ranging from severe neuropathic forms to attenuated, chronic visceral forms. Manifestations of the chronic visceral subtype are variable and encompass different degrees of hepatosplenomegaly, pulmonary disease and dyslipidemia. The aim of this study was to provide insights into the natural course of adult patients with the chronic visceral subtype. Based on these insights, we proposed tentative criteria for initiation and follow‐up of enzyme replacement therapy (ERT). The data of 23 adult patients were collected in a prospective study. Clinical, genetic and demographic data, plasma measurements, abdominal imaging, pulmonary imaging, pulmonary function tests and quality of life questionnaires were collected. Stability of disease based on several clinical, biochemical and radiological markers (i.e., spleen volume, platelet levels, liver volume, alanine aminotransferase [ALT] levels, diffusion capacity of the lungs for carbon monoxide [DLCO] chitotriosidase activity and lysosphingomyelin [LSM]) was assessed. Cardiovascular risk was estimated based on sex, age, smoking, systolic blood pressure and lipid profile. Quality of life was evaluated with the 36‐Item Short Form Health Survey and the Health Assessment Questionnaire. Median follow‐up was 6.1 years (range 1.3–19.5 years). The most common manifestations were splenomegaly (100%), decreased high‐density lipoprotein cholesterol (HDL‐C) plasma levels (83%), (signs of) steatosis measured with transient elastography (82%), thrombocytopenia (64%), hepatomegaly (52%) and decreased diffusion capacity (45%). The majority of markers remained stable during follow‐up. Twelve patients showed progression of disease: four for spleen volume, two for liver volume, three for DLCO, seven for chitotriosidase activity and three for LSM. One patient showed progression of disease based on four markers, although this patient did not report any problems at the last visit. Cardiovascular risk was estimated and was increased in half of the patients older than 40 years. Patient‐reported quality of life did not differ from the general population, but differences in median 36‐Item Short Form Health Survey (SF‐36) scores of patients with severe pulmonary involvement and those of patients without pulmonary involvement were observed. Tentative criteria for initiation and effect of therapy were proposed. In conclusion, the chronic visceral subtype of ASMD showed a predominantly stable disease course in this cohort. We propose that ERT should be initiated on an individual basis and only in case of progression or symptomatic disease. Collection and analysis of real world data are necessary to refine start, stop and follow‐up criteria in the future.
Keywords: biomarkers, chronic visceral ASMD, enzyme replacement therapy, start criteria
1. INTRODUCTION
Acid sphingomyelinase deficiency (ASMD, OMIM 607616), also known as Niemann‐Pick disease types A, A/B and B, is an ultra‐rare lysosomal storage disease with an estimated prevalence of 1 per 200 000 births. 1 , 2 Due to mutations in the SMPD1 gene, the enzyme acid sphingomyelinase (ASM, EC 3.1.4.12) is deficient which hampers conversion of sphingomyelin into ceramide. 3 This results in storage of sphingomyelin, predominantly in macrophages.
The diagnosis can be established through the demonstration of deficient enzyme activity in leucocytes or fibroblasts and additional mutation analysis. ASMD encompasses a broad spectrum of phenotypes, ranging from the severe infantile neurovisceral form, which typically proves to be fatal before the age of three and comprises severe neurological deterioration, to the chronic neurovisceral form characterized by moderate to severe manifestations, and the chronic visceral form without neurological involvement. 4 , 5 The most commonly affected organs are the spleen, liver and lungs. Splenomegaly, primarily attributed to sphingolipid accumulation, is seen in nearly all patients and may lead to thrombocytopenia or an increased risk of bleeding. 6 , 7 Hepatomegaly may progress to liver fibrosis, with rare cases exhibiting compromised liver function or cirrhosis. 8 Pulmonary involvement is characterized by sphingomyelin storage primarily within inter‐ and intralobular septae, can hamper diffusion capacity and may lead to dyspnea (mainly on extortion). 9 , 10 Additionally, patients show disrupted lipid profiles with decreased high‐density lipoprotein cholesterol (HDL‐C) and elevated low‐density lipoprotein cholesterol (LDL‐C) potentially increasing their risk of cardiovascular events. 11 Skeletal involvement (e.g., decreased bone mineral density and decreased fat fractions in bone marrow) has been reported. 12 , 13 However, its clinical relevance in adults with the chronic visceral subtype is debated as bone complications commonly observed in Gaucher disease (OMIM #230800), another lysosomal storage disorder with similar manifestations, do not appear to manifest in ASMD patients.
Until recently, no therapeutic options were available for ASMD. Enzyme replacement therapy (ERT) utilizing recombinant acid sphingomyelinase (olipudase alfa TM, Sanofi, MA, USA, NCT02004691) received market authorization by the European Medicines Agency and Food and Drug Administration in 2022. 14 , 15 Preclinical studies have demonstrated promising outcomes in adults with ASMD showing reversibility in the most common manifestations including significant reductions in hepatosplenomegaly and increase in diffusion capacity, along with improvements in plasma platelet levels, lipid profiles and liver transaminases. 16 , 17
However, due to the absence of long‐term follow‐up data of patients treated with ERT and the variability in ASMD phenotypes, treatment decisions should be made with care. The precise “window of opportunity” for initiating treatment remains uncertain. On the one hand, there is a preference for initiating treatment early to avert irreversible damage. On the other hand, there is a need to refrain from treatment in patients with stable and/or mild disease because ERT is burdensome and costly. Particularly regarding adult patients with relatively mild manifestations, contemplation about the benefit of treatment is imperative.
The aim of this study was to provide insights into the natural course of ASMD in untreated adult patients with the chronic visceral subtype by analysis of clinical and biochemical markers. Stability and progression of disease were assessed and formed a first step to arrive at criteria for initiation and follow‐up of ERT in these patients. Furthermore, cardiovascular risk and quality of life were investigated.
2. METHODS
2.1. Recruitment of patients
All adult patients known at the outpatient clinic of the Amsterdam UMC with a confirmed diagnosis of the chronic visceral subtype of ASMD (defined as decreased acid sphingomyelinase activity in leucocytes or fibroblasts, a pathogenic mutation in both alleles of the SMPD1 gene and the absence of neurological involvement) were eligible for this study. The Amsterdam UMC serves as the national referral center for ASMD in the Netherlands. All patients who were monitored between 2009 and October 2023 and provided written consent for the use of clinical and biochemical data were included in our prospective natural history study. Eight of them were described previously. 13 If available, plasma measurements (i.e., liver transaminases and lipid profiles) and pulmonary function test results conducted in other centers were included as well. Two patients participated in the phase II/III clinical trial with olipudase alfa (NCT02004691). Of them only data collected before the start of the clinical trial were included. The collection of clinical data within this study was approved by the local Medical Ethics Committee of the Amsterdam University Medical Centers, location Academic Medical Center (2014_092#A201468).
2.2. Collection of biochemical data
The following data were collected: clinical, genetic and demographic data, routine blood tests (e.g., hematological parameters, liver enzymes and lipid profiles), biochemical markers (i.e., chitotriosidase activity and chemokine C‐C motif ligand 18 [CCL18], lysosphingomyelin [LSM], N‐palmitoyl‐phosphocholineserine [PPCS, also known as LSM‐509] and glycoprotein nonmetastatic melanoma protein B [GPNMB]), abdominal imaging, pulmonary imaging and pulmonary function tests.
Plasma chitotriosidase activity and plasma levels of CCL18, LSM, PPCS and GPNMB were measured as previously described. 18 , 19 , 20 , 21 CHIT1 phenotypes were determined for all patients. In case of heterozygosity for the 24‐bp duplication in the CHIT1 gene, chitotriosidase activity was multiplied by two. 22 In case of homozygosity, patients were chitotriosidase deficient and excluded from analysis where appropriate. Local reference ranges for plasma measurements are provided in the supplemental methods. For HDL‐C and LDL‐C age and sex‐adjusted reference ranges were used, 23 decreased HDL‐C was defined as <5th percentile adjusted for age and sex and increased LDL‐C was defined as >95th percentile adjusted for age and sex.
2.3. Collection of clinical data
Data on hepatosplenomegaly were acquired through imaging techniques (preferably magnetic resonance imaging [MRI], in some cases ultrasound [e.g., patients with claustrophobia]). Precise measurements of spleen and liver volumes were feasible only with MRI. In cases where ultrasound was used, the report indicated the presence of splenomegaly or hepatomegaly. Organ volumes were adjusted to body weight and expressed in multiples of normal (MN) assuming normal spleen volume of 0.2% and normal liver volume of 2.5% of body weight with 1 kg equivalent to 1 L. 6 Spleen and liver volumes in MN were used to express splenomegaly and hepatomegaly. For the other analyses (e.g., correlation analysis and mixed‐effect models, see below), absolute volumes in milliliters were used, since spleen and liver volumes are only partially dependent of weight in adults.
Since 2020 Transient Elastography (TE) has been conducted within this cohort. TE measures liver stiffness and utilizes the controlled attenuation parameter (CAP) to evaluate both liver steatosis and fibrosis. Based on the guidelines for metabolic dysfunction‐associated steatotic liver disease (MASLD), elevated CAP was defined as >248 dB/m, indicating the presence of steatosis. A liver stiffness measurement above 7.1 kPa suggests the presence of fibrosis. 24 , 25
Data on pulmonary involvement comprised high‐resolution computed tomography (HRCT) scans of the lungs and pulmonary function tests including diffusion capacity of the lungs for carbon monoxide (DLCO) and carbon monoxide transfer coefficient (KCO, which is the diffusion capacity corrected for the alveolar volume). Pulmonary volumes and diffusion capacity were considered normal when values were >80% of predicted. Moreover, for a subset of patients data of 6 min walking tests (6MWT) were available and included. Individual reference ranges were calculated based on sex, age, height and weight. 26
2.4. Cardiovascular risk assessment
Cardiovascular risk assessment included various parameters such as body mass index (BMI), blood pressure, smoking habit, lipid profiles, statin therapy and previous cardiovascular events. Risk of cardiovascular disease (i.e., infarction or stroke) and/or death within 10 years for patients without cardiovascular events was estimated using the U‐Prevent SCORE2. 27 A risk of more than 5% was considered increased. 27 , 28 Factors included in the score were sex, age, smoking, systolic blood pressure, total cholesterol, plasma HDL‐C and LDL‐C levels. The model has been exclusively applied to patients older than 40 years and HDL‐C levels >0.7 mmol/L, so the risk of patients younger than 40 years was not calculated and if patients had an HDL‐C level lower than 0.7 mmol/L, 0.7 mmol/L was entered in the calculator. For patients with prior cardiovascular events, the SMART score was used. 29
2.5. Assessment of quality of life
Patients were requested to fill out questionnaires related to their quality of life, specifically the 36‐Item Short Form Health Survey (SF‐36) and the Health Assessment Questionnaire (HAQ). The SF‐36 comprised 36 questions each worth 0–100 points distributed across eight domains (i.e., physical functioning, physical health, emotional problems, fatigue, emotional well‐being, social functioning, pain, general health). 30 The HAQ evaluated the capability to perform daily activities and was distributed across eight domains (i.e., dressing, arising, eating, walking, hygiene, reach, grip, activities). 31 The scoring system for both questionnaires is described in the supplemental methods. Scores were compared with the general population and for patients with splenic involvement (i.e., moderate splenomegaly >5 MN or platelet levels <100 × 109/L) to patients without splenic involvement (i.e., splenomegaly <5 MN and normal platelet levels), for patients with hepatic involvement (i.e., moderate hepatomegaly >1.25 MN or ALT levels >2 upper limit of normal [ULN]) to patients without hepatic involvement (i.e., liver volume <1.25 MN and normal ALT levels) and for patients with moderate pulmonary involvement (i.e., DLCO <60% of predicted) to patients without pulmonary involvement (i.e., DLCO >80% of predicted).
2.6. Criteria to assess natural disease course
Tentative criteria for progression of disease were defined for several markers available for this cohort based on validated criteria in similar diseases, specifically Gaucher disease, or analytical and intra‐individual variance in case of plasma measurements. No previously defined or validated criteria were available for ASMD. Criteria for chitotriosidase activity and LSM plasma levels were based on analytical variance and intra‐individual variance, as observed in our center. Criteria for organ volumes and diffusion capacity were based on previously defined stability criteria for Gaucher disease 32 and interstitial lung diseases. 33 Criteria for platelet and ALT measurements were based on analytical variance and intra‐individual variance. 34 , 35 Criteria were defined as follows:
an increase of 40% or more in chitotriosidase activity at any moment during follow‐up or two consecutive measurements both increasing more than 20%,
an increase of 20% or more in LSM plasma level at any moment during follow‐up or two consecutive measurements both increasing more than 10%,
an increase of 10% or more in spleen volume,
an increase of 10% or more in liver volume,
a decrease of 15% or more in DLCO,
a decrease of 20% or more in plasma platelet level, and
an increase of 35% or more in plasma ALT level.
Adult patients with a follow‐up within our center of more than 2 years were evaluated. Percentages are based on percentage of change of last measurement when compared with the baseline measurement. Only markers for which more than two measurements were available were included (with an exception for spleen and liver volume). Two consecutive measurements of plasma platelet levels or ALT levels had to be aberrant when compared with the baseline value to meet the criterion for progression. 32
2.7. Proposed criteria for initiation of ERT and effect of ERT
To formulate tentative criteria for the selection of adult ASMD patients with visceral disease manifestations who may benefit from ERT, we analyzed the results of the ASCEND study in relation to the outcomes of this longitudinal cohort and used cut off values defined and validated for related disorders (i.e., MASLD and interstitial lung disease [ILD]). The ASCEND‐trial was an international phase II/III, double‐blind, placebo‐controlled trial in which 36 patients were randomized 1:1 to receive ERT with olipudase alfa or placebo. 16 , 17 These results were also the basis for the tentative criteria for effect of therapy, that is, the minimally expected change based on the lower limit of the 95% confidence interval as reported in the results of the ASCEND trial.
2.8. Statistical analyses
Data were analyzed using R Studio version 4.2.1. Frequencies and descriptive statistics including median and range were computed for various variables. Medians and ranges from baseline visit or first measurement were reported. t‐Tests were used to compare SF‐36 scores between the ASMD cohort and the general population. p‐Values smaller than 0.05 were considered statistically significant.
Correlations were examined between clinical variables (i.e., spleen volume, plasma platelet levels, liver volume, plasma ALT levels and DLCO) and biochemical markers (i.e., chitotriosidase activity, CCL18, LSM, PPCS, and GPNMB). Reciprocal correlations were also assessed among biochemical markers. For each patient, markers from the most complete recent visit were selected and supplemented with markers measured within year. p‐Values were adjusted using the Benjamini–Hochberg procedure to control the false discovery rate.
To evaluate the effect of age on the level of several clinical markers (i.e., spleen volume, plasma platelet levels, liver volume, plasma ALT levels and DLCO), linear mixed‐effect models were tested. First, an unconditional means model was built with patient ID as a random effect (e.g., biomarker ~1, random = ~1|ID). Then, age was introduced to obtain an unconditional linear growth model (e.g., biomarker ~ age, random = 1|ID). These models were compared using the likelihood ratio test. Next the introduction of a random slope (e.g., biomarker ~ age, random = age|ID) was evaluated in the same way. Model assumptions were checked.
3. RESULTS
3.1. Patients
In total 23 patients, 13 males and 10 females, were included. Median follow‐up was 6.1 years (range 1.3–19.5 years). Median age at diagnosis was 25.3 years (range 2.6–64.1 years). Median age at first follow‐up was 31.7 years (range 17.3–64.2 years). No patients died during the study.
Twelve different variants in the SMPD1 gene were established, with four variants (p.Arg610del, p.Pro373Ser, p.Pro477Leu and p.Leu163Pro) observed in two or more patients. The p.Arg610del variant was the most common, accounting for 63% of the alleles. There are no known familial relations between patients in this cohort. Detailed characteristics and clinical manifestations of individual patients are presented in Table 1, whereas group characteristics are outlined in Table 2 and the course of several markers as described below is depicted in Figure 1.
TABLE 1.
Demography.
| ID | Sex | Classification | Age at diagnosis (years) | Age at first follow‐up (years) | SMPD1 variant | Enzyme activity (% of mean of ref. range) | Symptoms at first follow‐up | Decreased DLCO | Other clinical manifestations | |
|---|---|---|---|---|---|---|---|---|---|---|
| Splenomegaly | Hepatomegaly | |||||||||
| 1 | M | Chronic visceral | 20.5 | 26.5 | p.Leu551Pro/p.Leu551Pro | 13.8 (fibroblasts) | Yes | Yes | Yes | |
| 2 | F | Chronic visceral | 2.6 | 17.3 | p.Pro373Ser/p.Pro373Ser | 9.4 (fibroblasts) | Yes | Yes | No | |
| 3 | M | Chronic visceral | 19.9 | 31.2 | p.Arg610del/p.Arg610del | 13.8 (fibroblasts) | Yes | No | No | |
| 4 | M | Chronic visceral | 25.3 | 25.4 | p.Arg610del/p.Arg610del | 4.0 (fibroblasts) | Yes | No | Yes | |
| 5 | M | Chronic visceral | 34.7 | 35.0 | p.Arg610del/p.Arg610del | 7.6 (leucocytes) | Yes | Yes | Yes | Proteinuria with progressive decline of renal function and sphingomyelin accumulation in renal biopsy (Eskes et al. 60 ) |
| 6 | F | Chronic visceral | 37.8 | 50.3 | p.Arg610del/p.Arg610del | 9.6 (fibroblasts) | Yes | No | Yes | |
| 7 | M | Chronic visceral | 4.9 | 19.4 | p.Arg610del/p.Val557IlefsX19 | 2.3 (fibroblasts) | Splenectomy | Yes | No | |
| 8 | M | Chronic visceral | 35.4 | 34.6 | p.Arg610del/p.Arg610del | 10.7 (fibroblasts) | Yes a | Yes a | Yes | |
| 9 | M | Chronic visceral | 47.8 | 48.0 | p.Arg291His/p.Pro477Leu | 34.0 (fibroblasts) | Yes a | No a | No | |
| 10 | M | Chronic visceral | 40.9 | 40.0 | p.Ile178Asn/p.Arg341Pro | 3.2 (leucocytes) | Yes | No | No | |
| 11 | M | Chronic visceral | 47.3 | 47.4 | p.Leu163Pro/p.Leu163Pro | 6.5 (fibroblasts) | Yes | No | No | |
| 12 | F | Chronic visceral | 34.5 | 35.3 | p.Arg610del/p.Arg610del | 11.3 (fibroblasts) | Yes | Yes | Yes | |
| 13 | M | Chronic visceral | 18.4 | 18.0 | p.Arg610del/p.Arg610del | 9.3 (fibroblasts) | Yes | Yes | No | |
| 14 | F | Chronic visceral | 43.3 | 43.2 | p.Pro373Ser/p.Pro373Ser | 12.7 (leucocytes) | Yes | No | Yes | |
| 15 | F | Chronic visceral | 8.0 | 17.8 | p.Arg610del/p.Arg610del | 9.5 (leucocytes) | Yes a | Yes a | No | |
| 16 | M | Chronic visceral | 2.8 | 17.4 | p.Arg610del/p.Arg610del | 9.5 (leucocytes) | Yes a | Yes a | No | |
| 17 | F | Chronic visceral | 22.6 | 22.8 | p.Arg610del/p.Arg610del | 7.5 (fibroblasts) | Yes | No | Yes | |
| 18 | F | Chronic visceral | 64.1 | 64.2 | p.Arg610del/p.Arg610del | 2.5 (leucocytes) | Yes | No | No | |
| 19 | F | Chronic visceral | 4.7 | 19.0 | p.Leu103Pro/p.Leu103Pro | 1.3 (fibroblasts) | Yes a | No a | NA | Hardened skin lesions with sphingomyelin accumulation in skin biopsy |
| 20 | M | Chronic visceral | 31.5 | 31.7 | p.Leu163Pro/p.Leu163Pro | 10.1 (fibroblasts) | Splenectomy | No | Yes | |
| 21 | M | Chronic visceral | 37.3 | 38.3 | p.Val314Met/p.Val314Met | 21.4 (fibroblasts) | Yes | Yes | No | |
| 22 | F | Chronic visceral | 15.3 | 25.1 | p.Arg610del/p.Leu139Pro | 3.1 (leucocytes) | Yes a | Yes a | No | |
| 23 | F | Chronic visceral | 5.0 | 57.7 | p.Arg610del/p.Pro477Leu | 2.5 (leucocytes) | Yes a | Yes a | Yes | Coronary artery bypass grafting (three times) at the age of 61 |
Note: Patients' characteristics are depicted. Enzyme activity is depicted in fibroblasts if available. Symptoms are based on last follow‐up.
Abbreviation: DLCO, diffusion capacity of the lungs for carbon monoxide.
Established on sonography, since no MRI data available.
TABLE 2.
Group characteristics.
| Percentage of total or median (range) | Total n | |
|---|---|---|
| Female sex | 43% | 23 |
| Mutation | 23 | |
| R610del homozygous | 48% | |
| R610del heterozygous | 14% | |
| Other | 39% | |
| Enzyme activity (median % of mean of ref. range) | 23 | |
| Fibroblasts | 9.6 (1.3–34.0) | 15 |
| Leucocytes | 5.9 (2.5–12.7) | 8 |
| Splenic manifestations | ||
| Splenectomy | 9% | 23 |
| Splenomegaly | 100% | 21 |
| Thrombocytopenia | 64% | 22 |
| Anemia | 9% | 23 |
| Leukopenia | 9% | 23 |
| Hepatic manifestations | ||
| Hepatomegaly | 52% | 23 |
| Elevated ALT levels | 43% | 23 |
| Elevated AST levels | 43% | 23 |
| (Signs of) steatosis on fibroscan | 82% | 17 |
| (Signs of) fibrosis on fibroscan | 35% | 17 |
| Pulmonary manifestations | ||
| Decreased DLCO | 45% | 22 |
| Decreased FVC | 27% | 22 |
| Decreased KCO | 30% | 20 |
| Decreased distance walked in 6MWT | 27% | 11 |
| Lipid profiles | ||
| Decreased HDL‐C levels | 83% | 23 |
| Elevated LDL‐C levels | 35% | 23 |
Note: Characteristics are based on the baseline measurements of the markers per patient. Total n concerns the number of patients for whom data was available. Splenomegaly and hepatomegaly were based on magnetic resonance imaging (MRI) or ultrasound if no MRI was available.
Abbreviations: 6MWT, 6 min walking test; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLCO, diffusion capacity of the lungs for carbon monoxide; FVC, forced vital capacity; HDL‐C, high density lipoprotein cholesterol; KCO, carbon monoxide transfer coefficient; LDL‐C, low density lipoprotein cholesterol.
FIGURE 1.
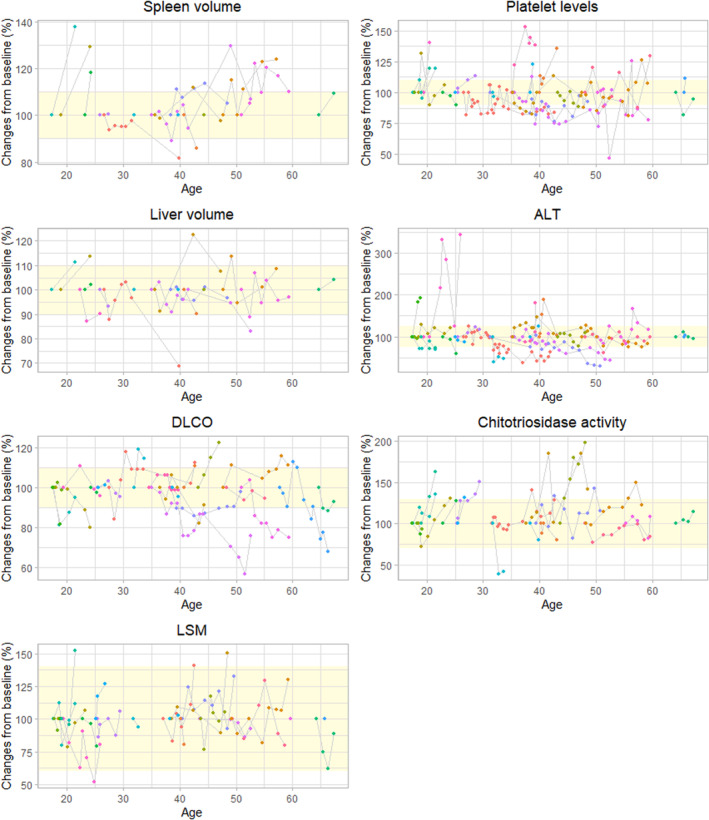
Markers during follow‐up per individual patient. The yellow square indicates the range within the marker is considered stable compared with baseline values. ALT, alanine aminotransferase; DLCO, diffusion capacity of the lungs for carbon monoxide; LSM, lysosphingomyelin.
3.2. Splenic manifestations and platelet levels
All patients had splenomegaly. Median spleen volume at first measurement was 1138 mL (range for median values 492–3076 mL) in 15 patients in whom volume was assessed by MRI. Two patients had a splenectomy, one because of massive spleen infarction with secondary infection after a trauma and the other due to recurrent splenic hemorrhage. On MRI and ultrasounds, multiple foci in the spleen were observed in nine patients, accessory spleens were observed in three patients. Multiple foci in the spleen have been described previously in ASMD patients, 36 the foci in these patients remained stable during follow‐up.
Median plasma platelet level at first visit was 135 × 109/L (range 81–259 × 109/L). The two patients who had a splenectomy showed increased platelet levels afterward (i.e., 484 × 109/L and 652 × 109/L). Fourteen patients (64%) had thrombocytopenia. Median hemoglobin levels for males were 15.3 g/dL (range 13.4–16.9 g/dL) and for females 13.5 g/dL (range 11.3–15.8 g/dL), with anemia present in two patients (9%). Two patients had decreased leucocyte levels at baseline which normalized during follow‐up.
3.3. Hepatic manifestations
Hepatomegaly was present in 12 patients (52%). Median liver volume was 2413 mL (range 1798–4772 mL, MRI data were available for 16 patients). Median plasma ALT level for males was 42 U/LL (range 14–128 U/L) and for females 30 U/L (range 12–146 U/L). Striking was an elevation in ALT levels during follow‐up of patients between the age of 25–30 (n = 4), in older patients levels were lower and similar to the levels of patients younger than 25 (elaborated on in section about mixed‐effect models below, depicted in Figure S1). Median plasma AST level was 31 U/L (range 18–129 U/L).
TE was performed in 18 patients. Median CAP score was 310 dB/m (range 170–400 dB/m). Median liver stiffness was 6.4 kPa (range 4.3–13.8 kPa), six patients (35%) showed (signs of) fibrosis based on a liver stiffness >7.1 kPa. Follow‐up was limited, so conclusions about stability or an upward or downward trend could not be drawn. No focal liver lesions were observed on MRI or ultrasound. One patient manifested gamna‐gandy bodies on MRI of the spleen, which is associated with portal hypertension, and increasing liver stiffness from 13.8 to 16.1 kPa, suggesting the onset of fibrosis. One patient with increased liver stiffness showed signs of portal hypertensive gastropathy and was referred to a hepatologist for further investigation.
3.4. Pulmonary manifestations
Pulmonary function testing was conducted for all patients, except for one patient because of technical issues. It is noteworthy that seven patients in this cohort were smokers. Median DLCO was 82% of predicted (range 28%–107% of predicted). Four patients showed a severely decreased DLCO during follow‐up of less than 40% of predicted of whom three had the habit of smoking. The individual course of DLCO per patient is shown in Figure S2 and illustrates the distinction between the four severely affected patients and the others.
Median forced vital capacity (FVC) was 97% of predicted (range 70%–129% of predicted). Median KCO was 93% of predicted (range 30%–124% of predicted). Nineteen patients showed signs of ILD on HRCT (n = 18) or x‐ray (n = 1) of the lungs, the other four patients did not show signs of ILD (for all HRCT was available). Most common findings on HRCT were thickened inter‐ and intralobular septae and ground glass opacities. Basal pulmonary areas were the most commonly and most severely affected.
At last visit, 3 of the 11 patients for whom 6MWT data were available walked a shorter distance than predicted. These were young patients (aged 18, 22 and 33 at testing). In contrast, the 6MWT results of three patients with severely decreased diffusion capacity were normal.
3.5. Lipid profiles
At first visit, the majority of the patients had decreased HDL‐C levels with a median of 0.7 mmol/L (range 0.4–1.1 mmol/L). Median LDL‐C plasma level was 3.2 mmol/L (range 0.3–8.0 mmol/L). Median total cholesterol was 4.9 mmol/L (range 2.4–9.6 mmol/L) and median triglyceride level was 2.0 mmol/L (range 0.8–7.4 mmol/L). The majority of patients (30%) had a combined dyslipidemia with decreased HDL‐C levels and increased LDL‐C levels. Nine patients used cholesterol lowering therapy (all statins). Notably, these patients commenced cholesterol‐lowering therapy at a relatively young age, with four of nine using statins before reaching the age of 40.
3.6. Biochemical markers
Plasma chitotriosidase activity levels were available for 20 patients, since three patients were deficient in chitotriosidase activity due to a homozygous 24 bp duplication in the CHIT1 gene. Six patients were heterozygous for this duplication. Median plasma chitotriosidase activity level was 1363 nmol/mL h (range 342–6620). LSM and PPCS plasma levels were measured in all patients, again with all measurements above the upper limit of the reference range. Median LSM level was 128 nmol/L (range 16–370). Median PPCS level was 1704 nmol/L (range 435–5240). Plasma CCL18 levels were available for 22 patients and were increased in all with a median of 459 ng/mL (range 104–894). GPNMB plasma levels were available for 17 patients. Median GPNMB level was 142 ng/mL (range 54–613 ng/mL).
3.7. Correlations and linear mixed‐effect models
No significant correlations were observed for several clinical and biochemical markers after correction for multiple testing (see Table S1).
Linear mixed‐effect models revealed no significant effect of age on spleen volume, platelet levels, liver volume and DLCO. A linear growth model with a random slope suggested an age‐related decrease in ALT levels; however, this trend appeared to be influenced by elevated levels in patients aged 25–30 years (n = 3). Two of these patients had a BMI >30 kg/m2. Since these elevated levels were not attributed to ASMD, the reliability of this fit was deemed questionable (see Figure S1). Additional models examining the effect of follow‐up time as independent variable, whereas correcting for age at first follow‐up did not show a significant effect either. Consequently, it can be concluded that these markers remained stable during follow‐up in this cohort. An overview of the models and their results is presented in Table S2.
3.8. Regression and progression of disease within the natural course
An overview of the course of several markers per individual patient is presented in Table 3. Five patients (29%) showed stability (or even regression) of disease based on all available markers, 11 patients (65%) showed stability when chitotriosidase activity and LSM were disregarded. Platelet levels and ALT levels were stable in all patients. Twelve patients (71%) showed progression of disease based on one or more markers: four for spleen volume, two for liver volume, three for DLCO, seven for chitotriosidase activity and three for LSM. One patient (patient 13) showed progression of disease based on four markers. This patient did not report any complaints at the last visit and did not experience limitations in daily activities. Furthermore it should be noted that this patient had mild manifestations based on the markers at baseline (see Table S3), with moderate splenomegaly (1550 mL, 12.3 MN), mildly decreased platelet levels (126 × 109/L), normal ALT levels and normal DLCO. Moreover, biochemical markers were relatively low compared with the other patients in this cohort, except for GPNMB. This patient and the other patient showing progression based on spleen and liver volume (patient 2) were first seen at our clinic after their transition from pediatric care. Potentially the increase in spleen and liver volume is (partly) due to growth. Their weight did not increase significantly during the follow‐up period (i.e., not more than 10%). Figure 2 illustrates the course of these markers for patient 13 and for patient 3, who showed stability for all markers during follow‐up.
TABLE 3.
Stability or spontaneous progression of disease based on changes in markers per individual patient.
| ID | Follow‐up in years | Spleen volume | Platelet levels | Liver volume | ALT levels | DLCO | Chitotriosidase activity | LSM |
|---|---|---|---|---|---|---|---|---|
| 1 | 16.3 | =/+ | = | =/+ | = | = | − | − |
| 2 | 2.8 | − | = | − | = | = | = | − |
| 3 | 12.4 | = | = | = | =/+ | = | = | = |
| 4 | 4.0 | = | = | = | = | = | − | = |
| 5 | 17.9 | = | = | =/+ | =/+ | − | Deficient | = |
| 6 | 8.7 | = | = | = | = | − | = | NA |
| 7 | 6.5 | Sx | = | = | = | = | Deficient | = |
| 8 | 5.7 | NA | =/+ | NA | = | = | NA | NA |
| 9 | 11.6 | NA | = | NA | = | = | = | − |
| 10 | 2.8 | = | = | =/+ | = | NA | = | NA |
| 11 | 11.4 | − | = | = | = | = | − | = |
| 12 | 12.9 | = | = | = | = | = | − | = |
| 13 | 6.1 | − | = | − | = | − | − | = |
| 14 | 5.8 | NA | = | NA | = | =/+ | − | = |
| 17 | 2.9 | − | = | = | = | = | = | = |
| 18 | 3.4 | = | = | = | = | = | = | = |
| 19 | 2.5 | NA | = | NA | = | NA | − | = |
Note: Only patients for whom sufficient data were available are displayed. Progression of disease, =: stable disease, =/+: spontaneous regression of disease (inverted criteria for progression of disease apply). Yellow: stable or improvement; red: deterioration; gray: data not available.
Abbreviations: ALT, alanine aminotransferase; DLCO, diffusion capacity of the lungs for carbon monoxide; LSM, lysosphingomyelin; NA, not available; Sx, splenectomy.
FIGURE 2.
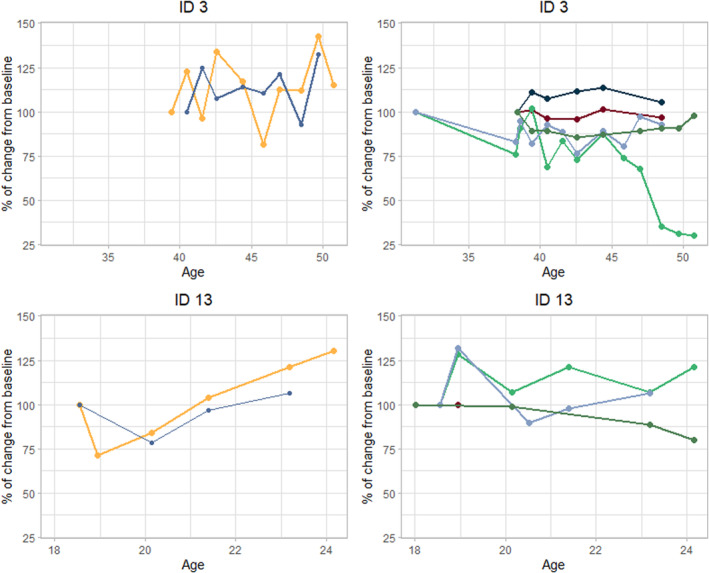
Individual course of markers in patient 3 and patient 13. Patient 3 showed stable disease (or spontaneous regression) for all markers (see Table 3). Patient 13 showed progression of disease based on spleen volume, liver volume, diffusion capacity of the lungs for carbon monoxide, chitotriosidase activity and lysosphingomyelin.
Notably, there was no clear relation between progression of disease regarding clinical markers and increase of biochemical markers (i.e., chitotriosidase activity and LSM). Some patients show progression based on a biochemical marker, but not for any clinical markers (i.e., patients 1, 4, 9, 12, 14 and 19). Only one patient (patient 1) showed progression based on both chitotriosidase activity and LSM plasma levels.
3.9. Cardiovascular risk
Cardiovascular risk was assessed individually for each patient, as detailed in Table 4. At the last visit, 16 patients were classified as overweight and four patients exhibited high blood pressure, with three already using antihypertensive medication, whereas the fourth was referred to the general practitioner to initiate medication. Seven patients (30%) were active smokers. Nine patients (39%) used statins and 20 patients (87%) had low HDL‐C plasma levels. 10‐Year risk of cardiovascular disease and/or death was calculated for 12 patients older than 40 years and was increased in six of them (50%). One of the patients underwent coronary artery bypass surgery, no other patients suffered cardiovascular events. For this patient, the 10‐year risk of recurrent cardiovascular disease and/or death was 20.3%.
TABLE 4.
Factors contributing to cardiovascular risk per patient.
| ID | Sex | Age at last follow‐up | BMI | Blood pressure at last follow‐up | Antihyper‐tensive medication | Smoking | Total chole‐sterol (mmol/L) | HDL‐C (mmol/L) | HDL‐C percen‐tile | LDL‐C (mmol/L) | LDL‐C percen‐tile | Statin | Age start statin | Cardio‐vascular event | Risk of cardiovascular disease and/or death a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 43.4 | 21.9 | 134/84 | No | No | 7.48 | 0.74 | 1 | 5.71 | 99 | Yes | 32.8 | No | 3.2% |
| 2 | F | 22.1 | 23.1 | 111/79 | No | No | 4.42 | 0.69 | 0 | 2.96 | 73 | Yes | 17.8 | No | |
| 3 | M | 50.8 | 28.8 | 130/80 | Yes | No | 3.18 | 0.69 | 1 | 1.86 | 1 | No | No | 3.1% | |
| 4 | M | 30.4 | 32.2 | 121/83 | No | Yes | 5.06 | 0.84 | 3 | 3.48 | 68 | No | No | ||
| 5 | M | 53.5 | 19.6 | 94/65 | Yes | Yes | 5.49 | 0.5 | 0 | 2.79 | 14 | Yes | 50.5 | No | 7.5% |
| 6 | F | 59.6 | 25.1 | 159/88 | Yes | Yes | 2.5 | 0.8 | 1 | 2 | 2 | Yes | 56.4 | No | 8.3% |
| 7 | M | 26.7 | 27.4 | 136/88 | No | No | 5.01 | 0.83 | 3 | 2.7 | 41 | No | No | ||
| 8 | M | 40.6 | 26.8 | 126/88 | No | No | NA | 0.83 | 3 | 4.52 | 88 | No | No | 2.6% | |
| 9 | M | 59.7 | 27.9 | 134/89 | Yes | No | 4.49 | 0.99 | 12 | 2.77 | 13 | No | No | 5.5% | |
| 10 | M | 44.6 | 28.4 | 175/110 | No (referred to GP) | No | 4 | 1.05 | 26 | 2.3 | 5 | No | No | 3.7% | |
| 11 | M | 59.7 | 29.4 | 136/80 | Yes | Yes | 4.19 | 0.72 | 1 | 1.56 | 1 | No | No | 9.4% | |
| 12 | F | 49.4 | 23.7 | 120/82 | No | Yes | 5.04 | 0.8 | 1 | 3.36 | 64 | No | No | 3.9% | |
| 13 | M | 24.6 | 22.0 | 132/76 | No | No | 2.75 | 0.54 | 1 | 0.88 | 1 | No | No | ||
| 14 | F | 49.3 | 33.8 | 119/80 | No | No | 3.88 | 1.01 | 3 | 2.53 | 20 | Yes | 43.2 | No | 1.5% |
| 15 | F | 19.1 | 26.2 | 96/66 | No | No | 4.9 | 0.72 | 0 | 3.14 | 79 | No | No | ||
| 16 | M | 19.4 | 24.9 | 134/89 | No | No | 2.44 | 0.5 | 0 | 1.08 | 1 | No | No | ||
| 17 | F | 26.5 | 35.5 | 112/85 | No | No | 3.8 | 0.89 | 2 | 2.43 | 39 | No | No | ||
| 18 | F | 68.0 | 32.7 | 146/85 | Yes | No | 4.65 | 0.89 | 1 | 2.86 | 11 | Yes | 65.4 | No | 7.2% |
| 19 | F | 22.4 | 21.5 | 113/78 | No | No | 3.83 | 0.6 | 0 | 2.41 | 40 | No | No | ||
| 20 | M | 34.1 | 27.5 | 124/76 | No | Yes | 6.67 | 1.21 | 42 | 4.9 | 97 | Yes | 32.8 | No | |
| 21 | M | 40.8 | 35.8 | 162/99 | Yes | Yes | 4.85 | 0.71 | 1 | 1.35 | 1 | No | No | 6.8% | |
| 22 | F | 27.0 | 35.5 | 106/69 | No | No | 4.51 | 0.91 | 2 | 2.92 | 67 | Yes | 22.0 | No | |
| 23 | F | 66.5 | 25.8 | 100/65 | Yes | No | 3.34 | 0.61 | 0 | 1.91 | 1 | Yes | Started <64 | Yes (CABG at age 61) | 20.3% b |
Note: All values are from patients' last visit. HDL‐C and LDL‐C percentiles are based on age and sex.
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; GP, general practitioner; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol.
10‐Year risk of cardiovascular disease and/or death according to the U‐Prevent SCORE2 using data on sex, age, smoking, systolic blood pressure, total cholesterol, plasma HDL‐C and LDL‐C levels.
One patient (patient 23) had a cardiovascular event, so 10‐year risk was calculated using the SMART score.
3.10. Quality of life
Median scores and ranges from the quality of life questionnaires are depicted in Table 5. Median Physical Component Summary (PCS) score from the SF‐36 questionnaire was 1190 (range 605–2435), median Mental Component Summary (MCS) score was 673 (range 250–1000). The SF‐36 scores of the ASMD patients from this cohort did not differ significantly from those in a healthy population (in which the mean PCS score was 1550 and the mean MCS score 705). 37 Comparison of patients with splenic involvement to those without showed slightly better PCS score for the patients with splenic involvement. Patients with hepatic involvement had lower SF‐36 scores than patients without. Median PCS score for patients with pulmonary involvement was almost a factor 3 lower than median PCS score for patients pulmonary involvement. MCS score showed a factor 2 difference.
TABLE 5.
Median scores and ranges for quality of life questionnaires (SF‐36 and HAQ).
| Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | |
|---|---|---|---|---|---|---|---|
| All patients | Splenic involvement | No splenic involvement | Hepatic involvement | No hepatic involvement | Pulmonary involvement | No pulmonary involvement | |
| SF‐36 | |||||||
| Amount of patients | 16 | 7 | 5 | 6 | 5 | 3 | 9 |
| Physical component summary | 1190 (605–2435) | 1910 (605–2320) | 1415 (625–2435) | 1435 (605–2300) | 1910 (755–2435) | 643 (605–800) | 1995 (625–2435) |
| Mental component summary | 673 (250–1000) | 730 (250–960) | 755 (295–1000) | 598 (250–840) | 900 (400–1000) | 388 (250–610) | 840 (265–1000) |
| HAQ | |||||||
| Amount of patients | 15 | 6 | 5 | 5 | 5 | 3 | 9 |
| Total | 0.13 (0–2.5) | 0 (0–0.63) | 0.13 (0–1.5) | 0 (0–0.63) | 0 (0–0.38) | 0.63 (0.25–1.63) | 0 (0–1.5) |
Note: Scores for SF‐36 can range from 0 to 2500 (PCS score) and from 0 to 1000 (MCS score). Score for HAQ can range from 0 to 3.
Abbreviations: HAQ, Health Assessment Questionnaire; MCS, Mental component summary; PCS, Physical component summary; SF‐36, 36‐Item Short Form Health Survey.
Median HAQ score was 0.13 (range 0–2.5), which is lower and thus better than the reported population mean (0.25 with a 95% confidence interval of 0.22–0.28). 38 Interestingly, patients without splenic involvement reported more disability than patients without as illustrated by a higher HAQ‐score. The median for both patients with and without hepatic involvement was zero. Patients with pulmonary involvement reported more disability.
3.11. Proposal for criteria for initiation and effect of treatment
Criteria for selection of adult ASMD patients with visceral disease manifestations who may benefit from ERT were proposed as follows:
plasma platelet levels <50 × 109/L OR,
spleen volume >12 MN or spleen volume inflicting substantial complaints impacting daily functioning OR,
liver stiffness >7.1 kPa (showing an upward trend over multiple measurements) or plasma ALT levels >2 ULN OR,
DLCO <70% of predicted OR, and
Evident progression of disease on any domain (even if criteria above are not met).
Any other cause of the changes as defined within the criteria should be excluded. Platelet levels were based on increased bleeding risk when platelets are <50 × 109/L. 39 Spleen volume was based on the data from this cohort (see Table S3). Liver stiffness was based on the cut off value used in MASLD. 25 Plasma ALT levels and DLCO cut off values were based on the ASCEND trial. 16 , 17
Criteria for effect of therapy were proposed based on the results of the international clinical trial of olipudase alfa. After 1 year of treatment, significant improvement was seen in the patients randomized to receive olipudase alfa for the following markers: chitotriosidase activity (decrease of 55%), LSM (decrease of 78%), spleen volume (decrease of 39%), liver volume (decrease of 28%), DLCO (increase of 22%), platelet level (increase of 17%), ALT level (decrease of 37%). 16 After 1 year, the placebo group started olipudase treatment, the results after 1 year of treatment for this group were similar. 17 Criteria for effect of therapy after 1 year of treatment were proposed as follows:
a reduction of 30% or more in chitotriosidase activity.
AND
a reduction of 50% or more in LSM plasma level.
AND ONE of the criteria below:
a reduction of 30% or more in spleen volume,
a reduction of 20% or more in liver volume,
an improvement of 15% or more in DLCO,
an increase of 10% or more in plasma platelet level (only if abnormal at start of treatment), and
a reduction of 15% or more in plasma ALT level (only if abnormal at start of treatment).
Decrease of chitotriosidase activity and LSM should be achieved within a year as well as one other criterion. For plasma markers, the effect is considered sustained if two consecutive measurements meet the criteria. The proposed criteria reflect the minimal expected change based on the lower limit of the 95% confidence interval as reported in the results of the international olipudase trial. 16 No patient in this cohort met these effect criteria during follow‐up.
4. DISCUSSION
In summary, the most common manifestations encompassed splenomegaly, decreased HDL‐C plasma levels, (signs of) steatosis measured with TE, thrombocytopenia, hepatomegaly and decreased DLCO. The majority of the markers measured in adult patients with chronic visceral ASMD were stable during follow‐up. Although cardiovascular risk was increased in half of the patients aged over 40 years, only one recorded cardiovascular event was observed within our population. Patient‐reported quality of life did not differ from the general population, although a difference in median SF‐36 scores was observed when comparing of patients with severe pulmonary involvement to those of patients without pulmonary involvement.
The most common disease manifestations in this cohort were in accordance with previously described cohorts of ASMD patients. 5 , 6 , 13 , 40 , 41 , 42 However, it should be noted that our cohort differs from most others because it only comprises adults with the chronic visceral subtype of ASMD. Moreover the p.Arg610del mutation was the most prevalent, accounting for 63% of the alleles, consistent with prior research indicating its association with the chronic visceral subtype of ASMD. 43 This mutation was associated with a relatively mild course of disease in previous studies, 44 , 45 especially less organomegaly, therefore findings in this cohort might be more favorable than in other cohorts of chronic visceral adults. This cohort was too small to investigate genotype–phenotype correlations, specifically in the context of disease progression, but it may prove important based on future research.
There are several observations that warrant further discussion. First, the distinction between patients with severe pulmonary disease (i.e., DLCO <40% of predicted, n = 4) and the other patients. It should be noted that three of these four patients were smokers, a habit normally expected to moderately influence DLCO. 46 In previous studies, several adult ASMD patients were described to have severely decreased DLCO, but no hypotheses regarding specific factors that could contribute to severe lung disease were raised. 6 , 47 , 48 , 49 When comparing our four patients with severe pulmonary involvement to the patients without, no differences in FVC, chitotriosidase activity or plasma levels of CCL18, LSM, PPCS or GPNMB were found. This observation prompts the hypothesis that an individual predisposition, such as genetic factors or environmental elements like smoking, may contribute to severe pulmonary involvement in a subset of adult patients with the chronic visceral form of ASMD.
Second, median KCO values were higher than DLCO values. Some patients exhibited normal KCO measurements while also showing decreased DLCO values. This discrepancy has been described in patients with interstitial lung disease. 50 , 51 Moreover, most patients in this cohort demonstrated normal FVC, as described in previous case reports of adult ASMD patients. 48 , 49 , 52 Another natural history study reported decreased FVC in 47% of patients, however patients in this cohort had a median age of 17.6 years and were therefore potentially more severely affected than the adult patients in our cohort. 53 Of note, three of the four patients with severe pulmonary involvement had normal FVC, the fourth had a mildly decreased FVC of 78% of predicted. This prompts the hypothesis that sphingomyelin storage in the lung hampers diffusion capacity, but does not affect pulmonary compliance to the same extent.
Furthermore, the 6MWT results are different than one might expect since three relatively young patients walked less distance than predicted based on age, sex, height and weight, whereas three older patients with severe pulmonary involvement reached the lower limit of normal within walking for 6 min. A potential explanation is the fact that the formulas to calculate predicted values are designed for patients older than 40 years. 26 Extrapolation of these formulas to younger patients might exceed a distance that is realistic to walk within 6 min. On the other hand, the patients with severe pulmonary involvement fell within the age range for which the formulas are designed. The fact that they were able to walk the distance defined as the lower limit of normal suggests that an effort as short as walking for 6 min is not impaired, as illustrated by a study in which only 5% of ASMD patients had 6MWT results lower than predicted. 53
Cardiovascular risk analysis showed that most patients had aberrant lipid profiles with most commonly decreased HDL‐C levels within a combined dyslipidemia, which was in line with previous research. 11 , 13 Half of the patients older than 40 years had a potential 10‐year risk for cardiovascular disease and/or death >5% based upon the standard criteria. Some patients showed disrupted lipid profiles at a young age, for instance a patient (patient 2) who had a LDL‐C plasma level of 5.5 mmol/L at the age of 18 years (99th percentile). Therefore exposure to atherogenic lipid profiles seems to be long‐term, as is observed in patients with genetic hyperlipidemias, possibly leading to increased cardiovascular risk. Therefore, patients in our cohort were prescribed cholesterol lowering therapy at a relatively young age: four of nine patients using cholesterol lowering therapy started before the age of 40, which lowered the LDL‐C plasma levels of aforementioned patient (patient 2) to the 73rd percentile in 2 years. In the recently published guideline for ASMD, the majority of experts agreed with the statement that ASMD patients might be at an increased risk of premature cardiovascular events because of aberrant lipid profiles. 54 However, it was noted that an increased cardiovascular risk has not been proven in ASMD and therefore the prescription of cholesterol lowering therapy warrant careful consideration based on individual characteristics. 54 Although there was only one cardiovascular event during follow‐up of the 23 patients of this cohort, the risk calculation performed in this study suggests an increased cardiovascular risk in a subset of patients. Further studies investigating cardiovascular events and risk in larger cohorts of adult ASMD patients are warranted.
Lastly, findings regarding quality of life are of interest. SF‐36 and HAQ scores of this cohort were similar to the general population. However, a decrease of quality of life was observed when patients with pulmonary involvement were compared with those without. This might suggest that pulmonary involvement, and decreased DLCO in particular, is one of the most debilitating features of ASMD in adults, as was suggested previously. 13 , 53 Nonetheless, it should be noted that ASMD is a chronic disease and patients might have adjusted to the limitations they experience, which might be illustrated by the fact that the quality of life questionnaire (i.e., the splenomegaly‐related score [SRS]) used in the ASCEND trial did not show a decrease after 1 year of treatment. Moreover, neither the SF‐36 nor the HAQ questionnaire was not validated for adult patients with ASMD. A validated questionnaire focusing on specific manifestations of ASMD and their influence on the quality of life of ASMD patients would be a convenient tool to periodically evaluate quality of life as experienced by patients and could aid in evaluating effect of ERT as well.
Based on this cohort, clinical manifestations of ASMD in adult seem to be compartmentalized. For example, some patients show severe pulmonary disease with limited splenomegaly, whereas others show hepatomegaly with increased liver enzymes but normal pulmonary function. No correlations were found between biochemical markers (considered to reflect general burden of disease) and markers reflecting splenic, hepatic or pulmonal involvement (see Table S3). Moreover most patients who showed progression of disease based on a clinical marker, did not show progression based on biochemical markers and conversely, not all patients who showed progression based on chitotriosidase activity showed progression of disease based on a clinical marker. Although biochemical markers do seem to reflect general burden of disease since they are increased in all patients and chitotriosidase activity and LSM levels decrease drastically after 1 year of ERT, these markers do not seem to predict progression of organ‐specific manifestations.
The aim of ERT is to alleviate disease burden and to prevent irreversible disease manifestations, for instance splenectomy. The indications for splenectomy in this cohort were spleen hemorrhage and infarction. Unfortunately, studies describing risk factors or clinical features of patients who underwent splenectomy (e.g., spleen volume before splenectomy) were not identified. This lack of knowledge poses a challenge in reliably identifying patients at risk for this complication. Additionally, the border between reversibility and irreversibility concerning hepatic and pulmonary involvement has not yet been elucidated. These manifestations might be largely reversible. Sphingomyelin accumulation in liver tissue was almost entirely cleared after 1 year of ERT and all patients with a DLCO of 40% of predicted or higher at baseline showed improvement of pulmonary function after 1 year of ERT. 16 , 17 TE emerges as a promising technique for assessing liver fibrosis, due to its non‐invasive nature. Liver involvement in ASMD resembles MASLD because both diseases are characterized by lipid accumulation in the liver and exhibit a broad spectrum of liver involvement, potentially leading to fibrosis or cirrhosis. 8 , 55 In MASLD, TE is widely used, because CAP score and measurements of liver stiffness have demonstrated correlation with liver biopsy results. 56 Lastly, hepatocellular carcinoma might be a complication of sphingomyelin storage in ASMD, as is the case in Gaucher disease. 57 However, scientific reports on liver lesions in ASMD are scarce. Focal liver lesions in ASMD patients were described in two case reports, 58 , 59 in both cases no hepatocellular carcinoma was established. A study describing the causes of death in ASMD patients mentions two patients with liver cancer, which is not further specified. 40
Because of the differences between individual patients and the uncertainty regarding irreversibility of manifestations, initiation of ERT should preferably be discussed in a multidisciplinary team and should be carefully considered for each individual patient. The tentative criteria proposed in this study (i.e., criteria for progression of disease, start criteria and effect criteria) might be of aid in this process. It should be noted that the criteria proposed in this study are based on the markers available for this cohort and limited scientific data. For instance, markers for liver stiffness, pulmonary imaging and bone density could be added. A next step would be to obtain a more definitive consensus from a multidisciplinary expert panel to arrive at generally accepted start and stop criteria, based on the description of this cohort as well as other studies.
Twelve patients (52%) from this cohort met one or more of the proposed start criteria and might benefit from therapy. Seven patients met the criterion for decreased DLCO, five patients the criterion for liver stiffness, four for spleen volume and four for ALT levels. No patients had platelet levels below 50 × 109/L. Eleven patients from this cohort (48%) have such mild disease manifestations that initiation of therapy does not seem beneficial (yet). The potential advantages of ERT need to be carefully weighed against its drawbacks, including the impact of regular infusions on a patient's life and the associated costs. This cohort shows that in a subset of adults with the chronic visceral subtype treatment may not be imperative or, at the very least, there is time for a watchful‐waiting approach, because of the mild manifestations and the absence of progression of these manifestations.
In conclusion, The chronic visceral subtype of ASMD exhibits a predominantly stable disease course in this relatively mild adult cohort. Consequently, the decision to initiate ERT in individual patients should be made with careful consideration. Future research is needed to expand the cohort to include potentially more severely affected patients by collecting real world data and refining the criteria. It could focus on investigating correlations between markers and clinical events, as well as on delineating the boundary between reversible and irreversible manifestations. Advancements in understanding these aspects will contribute to more precise and tailored therapeutic decisions for individuals with the chronic visceral subtype of ASMD.
CONFLICT OF INTEREST STATEMENT
LvD, FMV, JMFGA and ABPvK have no competing interests to declare. ECBE is involved in pre‐marketing studies with Sanofi Genzyme and Protalix as a sub‐investigator. MMMGB has participated in a pre‐marketing study with Intrabio. BS was involved in premarketing studies with Chiesi and Reneo Pharmaceuticals. CEMH is involved in premarketing studies with Sanofi Genzyme, Protalix and Idorsia.
ETHICS STATEMENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
INFORMED CONSENT
Informed consent was obtained from all patients for being included in the study.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGEMENTS
The authors want to express gratitude to Martijn van der Lienden for his work regarding the GPNMB analyses.
Eskes ECB, van Dussen L, Brands MMMG, et al. Natural disease course of chronic visceral acid sphingomyelinase deficiency in adults: A first step toward treatment criteria. J Inherit Metab Dis. 2025;48(1):e12789. doi: 10.1002/jimd.12789
Communicating Editor: Robin Lachmann
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249‐254. [DOI] [PubMed] [Google Scholar]
- 2. Kingma SD, Bodamer OA, Wijburg FA. Epidemiology and diagnosis of lysosomal storage disorders; challenges of screening. Best Pract Res Clin Endocrinol Metab. 2015;29(2):145‐157. [DOI] [PubMed] [Google Scholar]
- 3. Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann‐Pick diseae. Proc Natl Acad Sci U S A. 1966;55(2):366‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wasserstein MP, Aron A, Brodie SE, Simonaro C, Desnick RJ, McGovern MM. Acid sphingomyelinase deficiency: prevalence and characterization of an intermediate phenotype of Niemann‐Pick disease. J Pediatr. 2006;149(4):554‐559. [DOI] [PubMed] [Google Scholar]
- 5. McGovern MM, Avetisyan R, Sanson BJ, Lidove O. Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD). Orphanet J Rare Dis. 2017;12(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGovern MM, Wasserstein MP, Bembi B, et al. Prospective study of the natural history of chronic acid sphingomyelinase deficiency in children and adults: eleven years of observation. Orphanet J Rare Dis. 2021;16(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eskes ECB, Sjouke B, Vaz FM, et al. Biochemical and imaging parameters in acid sphingomyelinase deficiency: potential utility as biomarkers. Mol Genet Metab. 2020;130(1):16‐26. [DOI] [PubMed] [Google Scholar]
- 8. Thurberg BL, Wasserstein MP, Schiano T, et al. Liver and skin histopathology in adults with acid sphingomyelinase deficiency (Niemann‐Pick disease type B). Am J Surg Pathol. 2012;36(8):1234‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillemot N, Troadec C, de Villemeur TB, Clement A, Fauroux B. Lung disease in Niemann‐Pick disease. Pediatr Pulmonol. 2007;42(12):1207‐1214. [DOI] [PubMed] [Google Scholar]
- 10. von Ranke FM, Pereira Freitas HM, Mancano AD, et al. Pulmonary involvement in Niemann‐Pick disease: a state‐of‐the‐art review. Lung. 2016;194(4):511‐518. [DOI] [PubMed] [Google Scholar]
- 11. McGovern MM, Pohl‐Worgall T, Deckelbaum RJ, et al. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145(1):77‐81. [DOI] [PubMed] [Google Scholar]
- 12. Wasserstein M, Godbold J, McGovern MM. Skeletal manifestations in pediatric and adult patients with Niemann Pick disease type B. J Inherit Metab Dis. 2013;36(1):123‐127. [DOI] [PubMed] [Google Scholar]
- 13. Hollak CE, de Sonnaville ES, Cassiman D, et al. Acid sphingomyelinase (ASM) deficiency patients in The Netherlands and Belgium: disease spectrum and natural course in attenuated patients. Mol Genet Metab. 2012;107(3):526‐533. [DOI] [PubMed] [Google Scholar]
- 14. (EMA) EMA . Xenpozyme 2022. Accessed May 10, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/xenpozyme
- 15. (FDA) FaDA . FDA approves first treatment for acid sphingomyelinase deficiency, a rare genetic disease 2022. Accessed May 10, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-acid-sphingomyelinase-deficiency-rare-genetic-disease
- 16. Wasserstein M, Lachmann R, Hollak C, et al. A randomized, placebo‐controlled clinical trial evaluating olipudase alfa enzyme replacement therapy for chronic acid sphingomyelinase deficiency (ASMD) in adults: one‐year results. Genet Med. 2022;24:1425‐1436. [DOI] [PubMed] [Google Scholar]
- 17. Wasserstein MP, Lachmann R, Hollak C, et al. Continued improvement in disease manifestations of acid sphingomyelinase deficiency for adults with up to 2 years of olipudase alfa treatment: open‐label extension of the ASCEND trial. Orphanet J Rare Dis. 2023;18(1):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voorink‐Moret M, Goorden SMI, van Kuilenburg ABP, et al. Rapid screening for lipid storage disorders using biochemical markers. Expert center data and review of the literature. Mol Genet Metab. 2018;123(2):76‐84. [DOI] [PubMed] [Google Scholar]
- 19. Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93(3):1288‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boot RG, Verhoek M, de Fost M, et al. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate marker for assessing therapeutic intervention. Blood. 2004;103(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 21. Eskes ECB, van der Lienden MJC, Sjouke B, et al. Glycoprotein non‐metastatic protein B (GPNMB) plasma values in patients with chronic visceral acid sphingomyelinase deficiency. Mol Genet Metab. 2023;139(4):107631. [DOI] [PubMed] [Google Scholar]
- 22. van Dussen L, Hendriks EJ, Groener JE, Boot RG, Hollak CE, Aerts JM. Value of plasma chitotriosidase to assess non‐neuronopathic Gaucher disease severity and progression in the era of enzyme replacement therapy. J Inherit Metab Dis. 2014;37(6):991‐1001. [DOI] [PubMed] [Google Scholar]
- 23. Balder JW, de Vries JK, Nolte IM, Lansberg PJ, Kuivenhoven JA, Kamphuisen PW. Lipid and lipoprotein reference values from 133,450 Dutch Lifelines participants: age‐ and gender‐specific baseline lipid values and percentiles. J Clin Lipidol. 2017;11(4):1055‐1064.e6. [DOI] [PubMed] [Google Scholar]
- 24. Leung PB, Davis AM, Kumar S. Diagnosis and management of nonalcoholic fatty liver disease. JAMA. 2023;330(17):1687‐1688. [DOI] [PubMed] [Google Scholar]
- 25. Zhang X, Wong GL, Wong VW. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(2):128‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Enright PL, Sherrill DL. Reference equations for the six‐minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384‐1387. [DOI] [PubMed] [Google Scholar]
- 27. Jaspers NEM, Blaha MJ, Matsushita K, et al. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J. 2020;41(11):1190‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dorresteijn JA, Visseren FL, Wassink AM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99(12):866‐872. [DOI] [PubMed] [Google Scholar]
- 30. Patel AA, Donegan D, Albert T. The 36‐item short form. J Am Acad Orthop Surg. 2007;15(2):126‐134. [DOI] [PubMed] [Google Scholar]
- 31. The Health Assessment Questionnaire (HAQ) disability index (DI) of the clinical health assessment questionnaire (version 96.4). Accessed May 10, 2024. https://www.niehs.nih.gov/research/resources/assets/docs/haq_instructions_508.pdf
- 32. de Fost M, Aerts JM, Groener JE, et al. Low frequency maintenance therapy with imiglucerase in adult type I Gaucher disease: a prospective randomized controlled trial. Haematologica. 2007;92(2):215‐221. [DOI] [PubMed] [Google Scholar]
- 33. Lammi MR, Baughman RP, Birring SS, et al. Outcome measures for clinical trials in interstitial lung diseases. Curr Respir Med Rev. 2015;11(2):163‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buoro S, Seghezzi M, Manenti B, et al. Biological variation of platelet parameters determined by the Sysmex XN hematology analyzer. Clin Chim Acta. 2017;470:125‐132. [DOI] [PubMed] [Google Scholar]
- 35. Carobene A, Braga F, Roraas T, Sandberg S, Bartlett WA. A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and gamma‐glutamyl transferase. Clin Chem Lab Med. 2013;51(10):1997‐2007. [DOI] [PubMed] [Google Scholar]
- 36. Jones SA, McGovern M, Lidove O, et al. Clinical relevance of endpoints in clinical trials for acid sphingomyelinase deficiency enzyme replacement therapy. Mol Genet Metab. 2020;131(1–2):116‐123. [DOI] [PubMed] [Google Scholar]
- 37. RAND C . 36‐item short form survey (SF‐36) scoring instructions. Accessed May 10, 2024. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html
- 38. Krishnan E, Sokka T, Hakkinen A, Hubert H, Hannonen P. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum. 2004;50(3):953‐960. [DOI] [PubMed] [Google Scholar]
- 39. Gauer RL, Whitaker DJ. Thrombocytopenia: evaluation and management. Am Fam Physician. 2022;106(3):288‐298. [PubMed] [Google Scholar]
- 40. Cassiman D, Packman S, Bembi B, et al. Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (Niemann‐Pick disease type B and B variant): literature review and report of new cases. Mol Genet Metab. 2016;118(3):206‐213. [DOI] [PubMed] [Google Scholar]
- 41. Lipinski P, Kuchar L, Zakharova EY, Baydakova GV, Lugowska A, Tylki‐Szymanska A. Chronic visceral acid sphingomyelinase deficiency (Niemann‐Pick disease type B) in 16 Polish patients: long‐term follow‐up. Orphanet J Rare Dis. 2019;14(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu J, Maegawa GHB, Zhan X, et al. Clinical, biochemical, and genotype‐phenotype correlations of 118 patients with Niemann‐Pick disease types A/B. Hum Mutat. 2021;42(5):614‐625. [DOI] [PubMed] [Google Scholar]
- 43. Wang R, Qin Z, Huang L, et al. SMPD1 expression profile and mutation landscape help decipher genotype‐phenotype association and precision diagnosis for acid sphingomyelinase deficiency. Hereditas. 2023;160(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wasserstein MP, Larkin AE, Glass RB, Schuchman EH, Desnick RJ, McGovern MM. Growth restriction in children with type B Niemann‐Pick disease. J Pediatr. 2003;142(4):424‐428. [DOI] [PubMed] [Google Scholar]
- 45. Simonaro CM, Desnick RJ, McGovern MM, Wasserstein MP, Schuchman EH. The demographics and distribution of type B Niemann‐Pick disease: novel mutations lead to new genotype/phenotype correlations. Am J Hum Genet. 2002;71(6):1413‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akgun KB, Ozge C, Tasdelen B. Evaluation of carbonmonoxide, diffusion capacity, respiratory muscle strength values, and pulmonary volume in smoking men over 40 years old. Turk Thorac J. 2021;22(4):311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mendelson DS, Wasserstein MP, Desnick RJ, et al. Type B Niemann‐Pick disease: findings at chest radiography, thin‐section CT, and pulmonary function testing. Radiology. 2006;238(1):339‐345. [DOI] [PubMed] [Google Scholar]
- 48. Minai OA, Sullivan EJ, Stoller JK. Pulmonary involvement in Niemann‐Pick disease: case report and literature review. Respir Med. 2000;94(12):1241‐1251. [DOI] [PubMed] [Google Scholar]
- 49. Iaselli F, Rea G, Cappabianca S, et al. Adult‐onset pulmonary involvement in Niemann‐Pick disease type B. Monaldi Arch Chest Dis. 2011;75(4):235‐240. [DOI] [PubMed] [Google Scholar]
- 50. Johnson DC. Importance of adjusting carbon monoxide diffusing capacity (DLCO) and carbon monoxide transfer coefficient (KCO) for alveolar volume. Respir Med. 2000;94(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 51. Pastre J, Plantier L, Planes C, et al. Different KCO and VA combinations exist for the same DLCO value in patients with diffuse parenchymal lung diseases. BMC Pulm Med. 2015;15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferretti GR, Lantuejoul S, Brambilla E, Coulomb M. Case report. Pulmonary involvement in Niemann‐Pick disease subtype B: CT findings. J Comput Assist Tomogr. 1996;20(6):990‐992. [DOI] [PubMed] [Google Scholar]
- 53. McGovern MM, Wasserstein MP, Giugliani R, et al. A prospective, cross‐sectional survey study of the natural history of Niemann‐Pick disease type B. Pediatrics. 2008;122(2):e341‐e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geberhiwot T, Wasserstein M, Wanninayake S, et al. Consensus clinical management guidelines for acid sphingomyelinase deficiency (Niemann‐Pick disease types A, B and A/B). Orphanet J Rare Dis. 2023;18(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. [DOI] [PubMed] [Google Scholar]
- 56. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717‐1730. [DOI] [PubMed] [Google Scholar]
- 57. Regenboog M, van Dussen L, Verheij J, et al. Hepatocellular carcinoma in Gaucher disease: an international case series. J Inherit Metab Dis. 2018;41(5):819‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strigaris K, Kokkinis K, Liberopoulos K, Kavvadias S, Tsouroulas M, Nikolacopoulou Z. Liver lesion on computed tomography and ultrasonography in adult Niemann Pick disease related to sea blue histiocyte syndrome – a case report. Hepatogastroenterology. 1993;40(3):240‐243. [PubMed] [Google Scholar]
- 59. Sechi A, Vit A, Avellini C, et al. Focal hepatic lesions in acid sphingomyelinase deficiency: differential diagnosis between foamy macrophages aggregates and malignancy. Mol Genet Metab Rep. 2021;29:100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eskes ECB, van der Lienden MJC, Roelofs J, et al. Renal involvement in a patient with the chronic visceral subtype of acid sphingomyelinase deficiency resembles Fabry disease. JIMD Rep. 2021;62(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


