Abstract
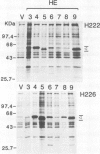
Site-directed mutagenesis was used to prepare a series of human oestrogen receptor (hER) deletion mutants. The ability of these mutant receptors to bind oestradiol, either after being transiently expressed in HeLa cells or produced synthetically in vitro using T7 polymerase coupled with a rabbit reticulocyte lysate translation system, was analysed. The results indicate that a region which is highly conserved (94% amino acid identity) between the human and chicken ERs (region E) contains all of the sequence necessary to bind oestradiol with high affinity. When tight nuclear association of the oestradiol-receptor complex was investigated using the oestradiol-binding mutants of the same series, two regions of the hER sequence were found to be important. One of these regions is completely conserved (100% amino acid identity) between the human and chicken ERs (region C). This region is rich in cysteine and basic amino acids and contains motifs similar to those which have been proposed to be important for DNA binding in other eukaryotic transcriptional regulatory proteins. The other region (region D), which is comparatively poorly conserved (38% amino acid identity), is located between the putative DNA-binding domain (region C) and the oestradiol-binding domain (region E).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Harris B. A. Plasmids for the cloning and expression of full-length double-stranded cDNAs under control of the SV40 early or late gene promoter. Nucleic Acids Res. 1983 Oct 25;11(20):7119–7136. doi: 10.1093/nar/11.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvard D. S., Wilson E. M. Zinc potentiation of androgen receptor binding to nuclei in vitro. Biochemistry. 1984 Jul 17;23(15):3471–3478. doi: 10.1021/bi00310a014. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Grundström T., Zenke W. M., Wintzerith M., Matthes H. W., Staub A., Chambon P. Oligonucleotide-directed mutagenesis by microscale 'shot-gun' gene synthesis. Nucleic Acids Res. 1985 May 10;13(9):3305–3316. doi: 10.1093/nar/13.9.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmar P. H., Toft D. O. Inhibition of the binding of progesterone receptor to nuclei: effects of o-phenanthroline and rifamycin AF/013. Biochem Biophys Res Commun. 1975 Nov 3;67(1):8–15. doi: 10.1016/0006-291x(75)90275-2. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Onishi T. Rapid isolation of nucleoli from detergent-purified nuclei of tumor and tissue culture cells. Methods Cell Biol. 1977;15:221–234. doi: 10.1016/s0091-679x(08)60218-6. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Sekula B. C., Litwack G. The effects of 1,10-phenanthroline on the binding of activated rat hepatic glucocorticoid-receptor complexes to deoxyribonucleic acid-cellulose. Endocrinology. 1981 Sep;109(3):803–812. doi: 10.1210/endo-109-3-803. [DOI] [PubMed] [Google Scholar]
- Shyamala G. Is the estrogen receptor of mammary glands a metallo-protein? Biochem Biophys Res Commun. 1975 May 5;64(1):408–415. doi: 10.1016/0006-291x(75)90268-5. [DOI] [PubMed] [Google Scholar]
- Walter P., Green S., Greene G., Krust A., Bornert J. M., Jeltsch J. M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrange O., Okret S., Radojćić M., Carlstedt-Duke J., Gustafsson J. A. Characterization of the purified activated glucocorticoid receptor from rat liver cytosol. J Biol Chem. 1984 Apr 10;259(7):4534–4541. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]