Abstract
The DNA sequence of the 5270-bp repeated DNA element from the mitochondrial genome of the fertile cytoplasm of maize has been determined. The repeat is a major site of recombination within the mitochondrial genome and sequences related to the R1(S1) and R2(S2) linear episomes reside immediately adjacent to the repeat. The terminal inverted repeats of the R1 and R2 homologous sequences form one of the two boundaries of the repeat. Frame-shift mutations have introduced 11 translation termination codons into the transcribed S2/R2 URFI gene. The repeated sequence, though recombinantly active, appears to serve no biological function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun C. J., Levings C. S. Nucleotide Sequence of the F(1)-ATPase alpha Subunit Gene from Maize Mitochondria. Plant Physiol. 1985 Oct;79(2):571–577. doi: 10.1104/pp.79.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Mendu N., Ginsburg H., Kridl J. C. Sequence analysis of the maize mitochondrial 26 S rRNA gene and flanking regions. Plasmid. 1984 Mar;11(2):141–150. doi: 10.1016/0147-619x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Dale R. M. Sequence homology among different size classes of plant mtDNAs. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4453–4457. doi: 10.1073/pnas.78.7.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., Timothy D. H. Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol. 1985 Nov;79(3):914–919. doi: 10.1104/pp.79.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac P. G., Brennicke A., Dunbar S. M., Leaver C. J. The mitochondrial genome of fertile maize (Zea mays L.) contains two copies of the gene encoding the alpha-subunit of the F1-ATPase. Curr Genet. 1985;10(4):321–328. doi: 10.1007/BF00365628. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Jones V. P., Leaver C. J. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 1985 Jul;4(7):1617–1623. doi: 10.1002/j.1460-2075.1985.tb03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Thompson R. D. S1 and S2, the linear mitochondrial DNAs present in a male sterile line of maize, possess terminally attached proteins. Nucleic Acids Res. 1982 Dec 20;10(24):8181–8190. doi: 10.1093/nar/10.24.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E. B., Angelides K. J., Holloman W. K. Left-handed DNA and the synaptic pairing reaction promoted by Ustilago rec1 protein. Cell. 1985 Jan;40(1):139–145. doi: 10.1016/0092-8674(85)90317-4. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Synapsis promoted by Ustilago rec1 protein. Cell. 1984 Mar;36(3):593–598. doi: 10.1016/0092-8674(84)90338-6. [DOI] [PubMed] [Google Scholar]
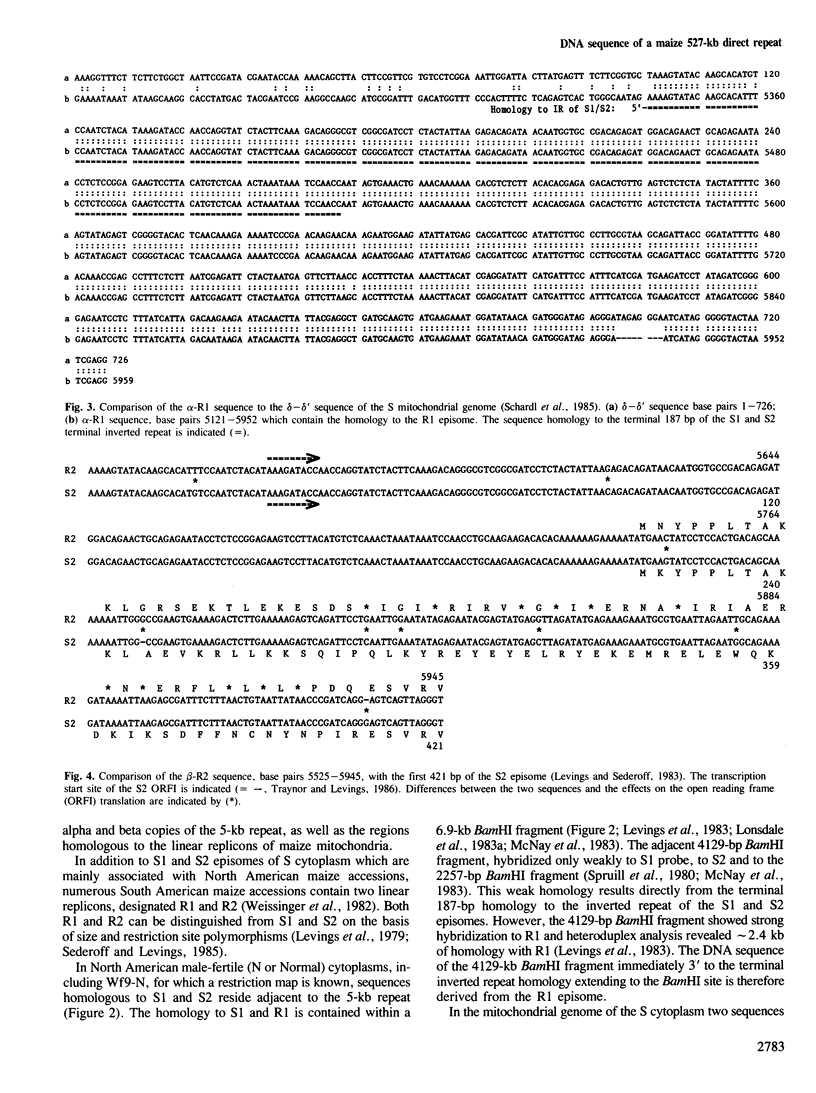
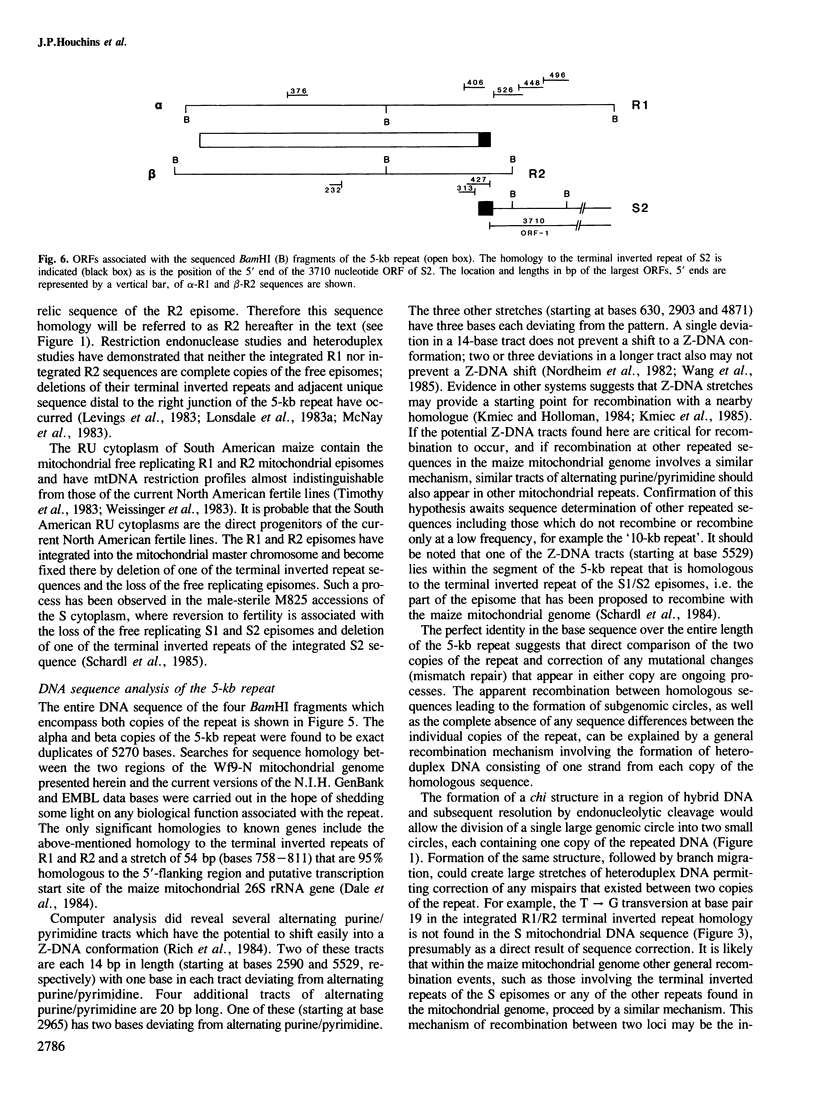
- Levings C. S., Sederoff R. R. Nucleotide sequence of the S-2 mitochondrial DNA from the S cytoplasm of maize. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4055–4059. doi: 10.1073/pnas.80.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
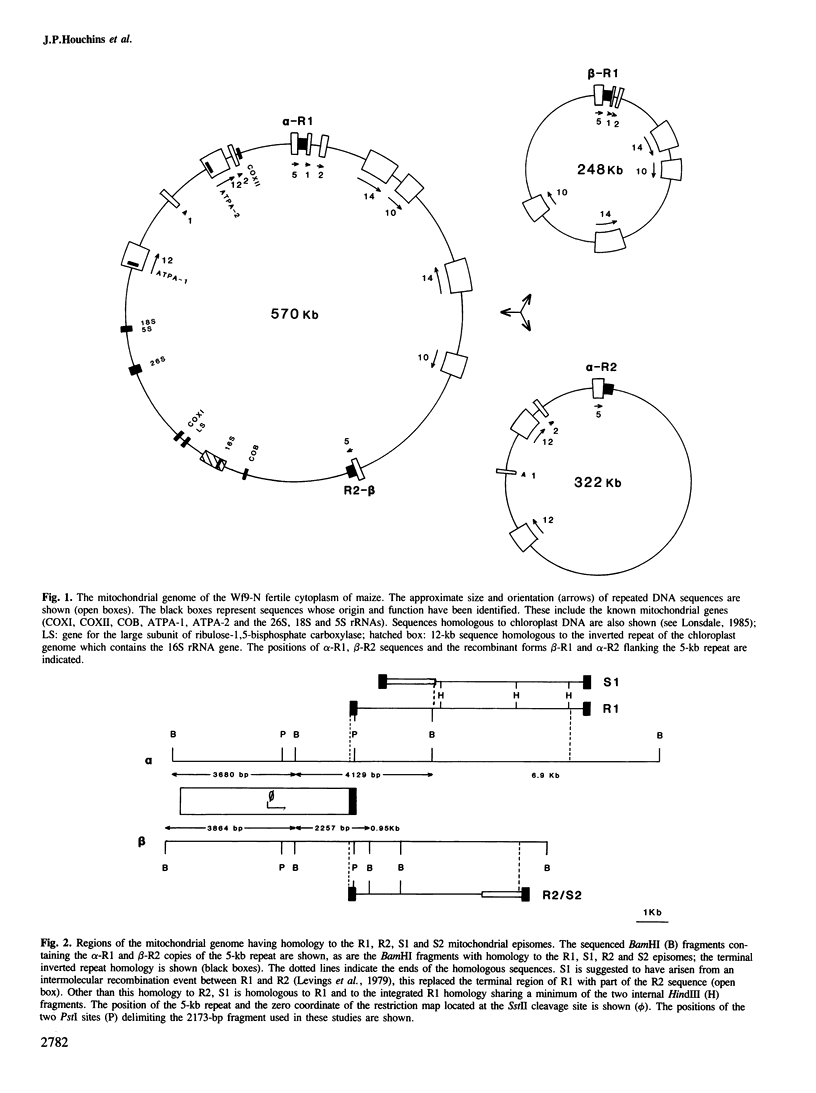
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Stoehr P. J. A computer program for the management of small cosmid banks. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):429–436. doi: 10.1093/nar/12.1part2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Paillard M., Sederoff R. R., Levings C. S. Nucleotide sequence of the S-1 mitochondrial DNA from the S cytoplasm of maize. EMBO J. 1985 May;4(5):1125–1128. doi: 10.1002/j.1460-2075.1985.tb03749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Rüther U. Construction and properties of a new cloning vehicle, allowing direct screening for recombinant plasmids. Mol Gen Genet. 1980;178(2):475–477. doi: 10.1007/BF00270503. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl C. L., Pring D. R., Lonsdale D. M. Mitochondrial DNA rearrangements associated with fertile revertants of S-type male-sterile maize. Cell. 1985 Nov;43(1):361–368. doi: 10.1016/0092-8674(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Thompson R. D., Kemble R. J., Flavell R. B. Variations in mitochondrial DNA organisation between normal and male-sterile cytoplasms of maize. Nucleic Acids Res. 1980 May 10;8(9):1999–2008. doi: 10.1093/nar/8.9.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Gessner R. V., van der Marel G. A., van Boom J. H., Rich A. Crystal structure of Z-DNA without an alternating purine-pyrimidine sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3611–3615. doi: 10.1073/pnas.82.11.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Weissinger A. K., Timothy D. H., Levings C. S., Goodman M. M. Patterns of mitochondrial DNA variation in indigenous maize races of latin america. Genetics. 1983 Jun;104(2):365–379. doi: 10.1093/genetics/104.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissinger A. K., Timothy D. H., Levings C. S., Hu W. W., Goodman M. M. Unique plasmid-like mitochondrial DNAs from indigenous maize races of Latin America. Proc Natl Acad Sci U S A. 1982 Jan;79(1):1–5. doi: 10.1073/pnas.79.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]