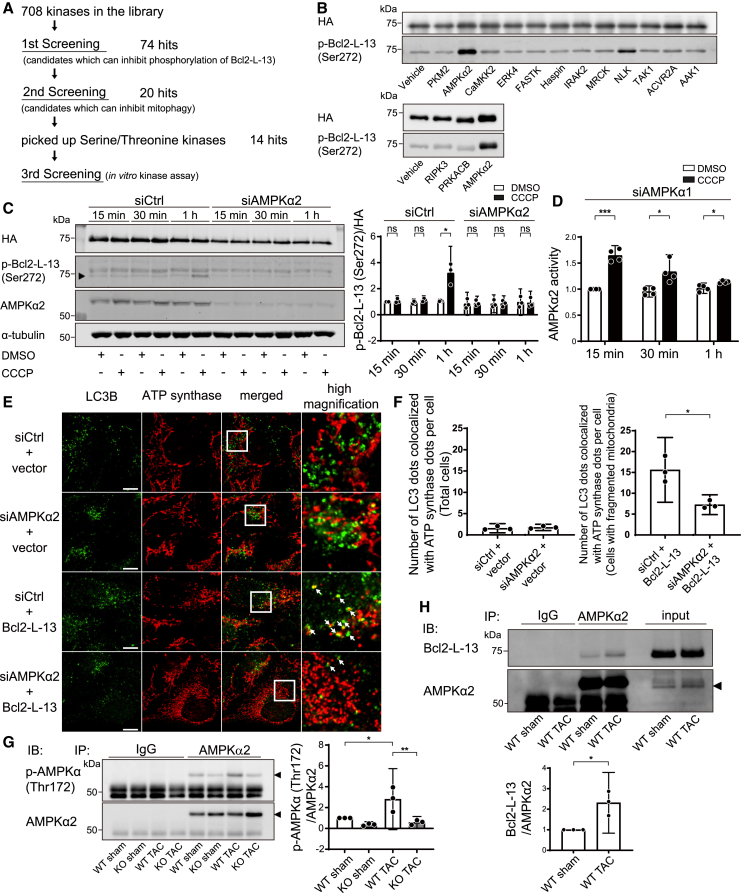
Figure 6.
AMPKα2 is the kinase responsible for Bcl2-L-13 phosphorylation at Ser272
(A) Schematic of the responsible kinase screening workflow.
(B) In vitro kinase assay in the third screening. Bacterially synthesized HA-Bcl2-L-13 was mixed with purified candidate proteins and ATP. After incubation at 37°C for 30 min, the reaction mix was subjected to western blotting using an anti-phospho-Bcl2-L-13 (Ser272) antibody.
(C) The effect of AMPKα2 knockdown in CCCP-induced Bcl2-L-13 phosphorylation. HEK293A cells stably expressing HA-Bcl2-L-13 were transfected with control siRNA or siAMPKα2 for 72 h. Then, the cells were treated with DMSO or 15 μM CCCP for the indicated times, and cell lysates were subjected to western blot analysis. Densitometric analysis of phospho-Bcl2-L-13 (Ser272) is shown in the bar graph. The value for the group with control siRNA (siCtrl) transfection and 15 min of DMSO treatment in each experiment was set to 1 (n = 3).
(D) Upregulation of AMPKα2 activity by CCCP treatment. To analyze AMPKα2-specific activity, HEK293A cells stably expressing HA-Bcl2-L-13 were transfected with siAMPKα1 for 72 h and then treated with DMSO or 15 μM CCCP. The value for the group with 15-min DMSO treatment in each experiment was set to 1 (n = 4).
(E and F) HEK293A cells were transfected with control siRNA (siCtrl) or siAMPKα2 for 72 h, followed by transfection with an empty vector or HA-Bcl2-L-13. Forty-four hours after transfection, cells were treated with 100 nM bafilomycin A1 for 4 h and immunostained with anti-LC3B and anti-ATP synthase antibodies. Images in the box at higher magnification are shown on the right. White arrows indicate the puncta recognized as colocalized by the software. The number of LC3B dots colocalized with ATP synthase dots per cell is shown in (F). At least 20 cells were counted for each group (n = 3). Scale bar: 10 μm.
(G) Upregulation of AMPKα2 phosphorylation 5 days after TAC operation. To analyze AMPKα2-specific phosphorylation, lysates from the left ventricle were subjected to immunoprecipitation with an anti-AMPKα2 antibody followed by immunoblotting with an anti-phospho-AMPKα (Thr172) antibody. Densitometric analysis of phospho-AMPKα (Thr172) is shown in the right bar graph. The value for the WT sham group in each experiment was set to 1 (n = 3).
(H) Interaction between Bcl2-L-13 and AMPKα2 5 days after TAC. Lysates from the left ventricle were subjected to immunoprecipitation with an anti-AMPKα2 antibody. Co-precipitated Bcl2-L-13 was detected by immunoblotting. Densitometric analysis of Bcl2-L-13 is shown in the graph below. The value for the WT sham group in each experiment was set to 1 (n = 3). Results are shown as mean with 95% CI. Statistical analysis by unpaired, two-tailed t tests in (C), (D), (F), and (H) and one-way ANOVA followed by Tukey-Kramer’s post hoc test in (G). All pairwise comparisons were performed. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. ns, not significant. See also Figures S6 and S7.