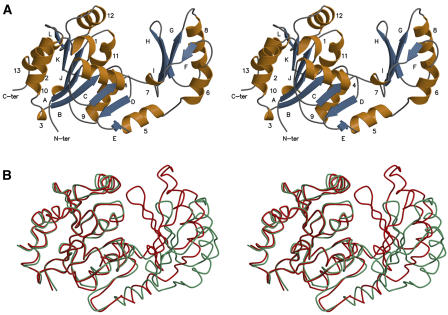
Figure 1.
Stereo View of the Ribbon Diagram of the Synechocystis SPP Fold.
The monomer contains 12 β-strands (A to L; blue) and 13 α-helices (1 to 13; gold).
(A) The open conformation of the protein.
(B) Superimposition of the two conformations (the open and closed ones are colored in green and red, respectively). The core domains were superimposed, showing the relative movement of the cap domain, which rotates by ∼25°.