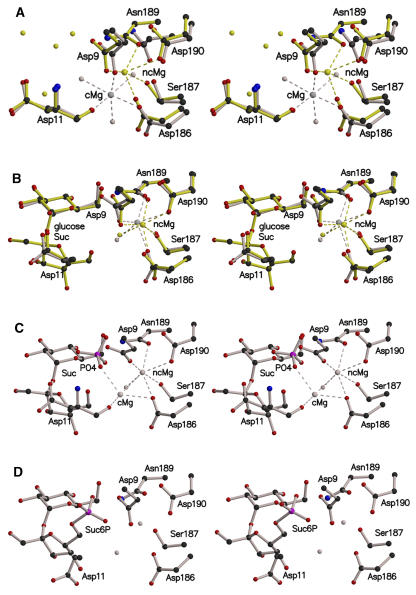
Figure 4.
Position of the Magnesium Ion in the Active Site of Synechocystis SPP (Stereo View).
Important residues in the active site are shown in ball-and-stick format.
(A) Superimposition of the open (gray) and closed (yellow) conformations of SPP, with a bound Mg2+ ion, showing the two positions that the metal can adopt. Mg2+ ions in the catalytic and noncatalytic positions are annotated as cMg and ncMg, respectively.
(B) Superimposition of SPP complexed with glucose (gray) or sucrose (yellow).
(C) Sucrose and phosphate bound in the active site after soaking of the closed conformation SPP crystal with Suc6P. The crystals contained a population of two magnesium ions, cMg and ncMg, with occupancies of 0.30 and 0.70, respectively.
(D) Active site of the SPP-Suc6P complex with no Mg2+ ion. Carbon, nitrogen, oxygen, and phosphorus atoms are colored in black, blue, red, and pink, respectively. Mg2+ ions and water molecules are shown in the same color as the protein in which they are complexed. Dashed lines indicate the coordination shell of the Mg2+ ion.