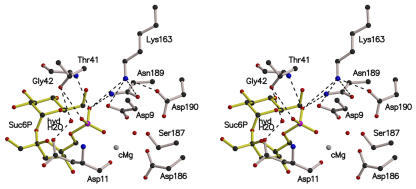
Figure 5.
Stereo View of the Active Site of the Synechocystis SPP in Complex with Its Substrate Suc6P.
The catalytic Mg2+ ion (cMg) and the hydrolytic water molecule (hydH2O) were modeled from the structures of the other SPP complexes and the deoxy-d-mannose-octulosonate 8-phosphate phosphatase from Haemophilus influenzae (Parsons et al., 2002). Dashed lines indicate possible hydrogen bonds. With this configuration, the nucleophilic Asp9 can attack the Suc6P at its phosphorus atom, leading to loss of the phosphate-sucrose bond and to formation of a phospho-Asp9-enzyme intermediate. After movement into the active site because of the steric environment, the hydrolytic water molecule could be in the right position to dephosphorylate the phospho-Asp9.