Abstract
Cerebral venous sinus thrombosis (CVST) is an uncommon and underreported neurological condition. This condition has varied aetiologies, clinical manifestations, and significant sequelae if left untreated. We report the case of a 10-year-old male with fever, altered sensorium, cranial nerve neuropathies, and left hemiplegia. His imaging findings revealed features consistent with CVST, and he was subsequently noticed to be passing live worms per rectum while on admission. He was managed nonoperatively, improved significantly, and was discharged with residual motor deficits. In addition, we present a literature review of published case reports of CVST associated with helminthiasis, highlighting its possible pathophysiology, clinical spectrum, and management peculiarities.
Keywords: Ascaris lumbricoides, cerebral venous sinus thrombosis, helminthiasis
Introduction
Cerebral venous sinus thrombosis (CVST) is the formation of clots within the dural sinuses. It is an infrequent cause of stroke affecting all age groups but commonly reported in the young adult population with a female predominance.[1] Paediatric CVST, a subset, has an incidence of about 0.6 per 100,000 children per year and mainly affects neonates.[2] Knowledge of paediatric CVST is still unfolding, with data from Africa mainly limited to case reports.[3] Common aetiological factors are infection, dehydration, and hypercoagulable state. Its clinical presentation is subtle and ranges from non-specific headache, anorexia, and vomiting to more sinister findings such as cranial neuropathies, hemiplegia, visual loss, cognitive deficits resulting in learning difficulties, and death.[4] Although the neurological sequelae of helminthiasis infestation have been extensively reported, CVST appears to be a rare occurrence with few published reports. Thus, a strong index of suspicion is needed for prompt diagnosis and the commencement of treatment.
Case Presentation
A 10-year-old right-handed male primary school pupil was referred from a private health facility due to right facial swelling lasting 9 days, accompanied by fever and altered sensorium for a week. The right facial swelling began as a painful boil near the eye and gradually extended to involve the right side of the face. Additionally, he experienced watery eye discharge and reduced movement of the same eye. Two days later, he developed a high-grade constant fever, which was mildly relieved by antipyretics, along with altered sensorium and left extremity weakness, progressing to an inability to use the left limbs. There were no reports of seizure, nausea, or vomiting.
There was no history of facial trauma or similar illness in the family. His genotype was unknown. He had no history of prior hospital admissions but was not fully immunised for his age. He is the third child in a monogamous family with seven offspring, residing in a shared apartment in a rural setting and drinks well water, with an open sewage and refuse disposal system.
A general examination revealed a chronically ill-looking male adolescent who was febrile with a temperature of 40°C and appeared pale. His weight was 19 kg (58% of expected, thus small for his age). There was a diffuse right-sided hemifacial erythematous swelling that was tender and firm, accompanied by periorbital oedema and matted eyelids with crust.
Neurological examination revealed a Glasgow Coma Score (GCS) of 10 (an eye-opening score of 3, a best verbal response of 2, and best motor response of 5). The right pupil measured 4 mm and showed sluggish reactivity to light, while the left pupil measured 3 mm and showed brisk reactivity to light, with normal fundoscopic findings. He had bilateral abducens nerve palsy with left upper motor neurone facial nerve palsy. The neck was persistently tilted to the right with marked stiffness, and positive Kernig and Brudniski signs were noted. He exhibited global reduced muscle bulk with left spastic hemiplegia. Other pertinent findings on systemic examination included generalised coarse crepitation and non-tender hepatomegaly, with the liver palpable 3 cm below the right costal margin.
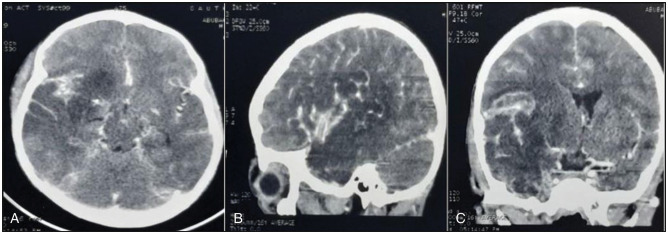
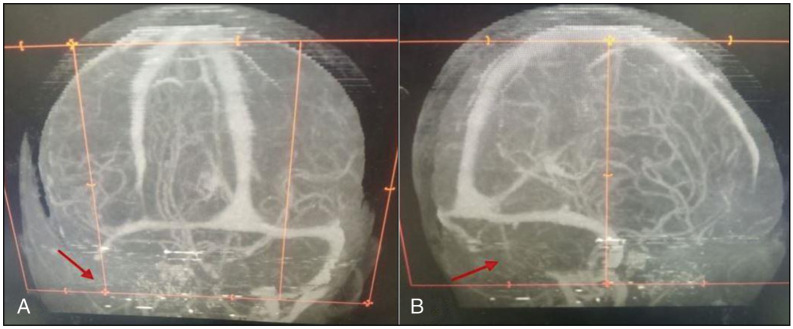
A cranial CT scan [Figure 1] revealed a right Sylvian fissure-based frontotemporoparietal hypodense lesion with mass effect, as evidenced by a marked midline shift with effacement of the ipsilateral ventricle. The vascular reformatted images [Figure 2] show complete right sigmoid sinus and internal jugular vein thrombosis (indicated by the red arrow), demonstrated by non-visualisation of the involved sinuses. These findings are consistent with cerebral venous thrombosis with venous infarct.
Figure 1.
A cranial CT scan revealed a right frontotemporoparietal hypodense lesion with mass effect as evidenced by a marked midline shift with effacement of the ipsilateral ventricle. These findings are in keeping with venous infarction. Axial, coronal, and sagittal views are shown in (a), (b), and (c), respectively
Figure 2.
The vascular reformatted images show a complete right sigmoid sinus and internal jugular vein thrombosis (red arrow) demonstrated by non-visualisation of the involved sinuses. These findings are in keeping with cerebral venous thrombosis. The coronal and sagittal views are shown in (a) and (b), respectively
His full blood count revealed a packed cell volume of 24% and leucocytosis of 14,500 cells/mm3 with neutrophilia. Serum electrolytes and urinalysis were normal. C-reactive protein was markedly elevated (176,000), while prothrombin time and activated partial thromboplastin time were 11.3 s (normal) and 47.9 s (elevated), respectively. The total serum protein was normal (74g/l), serum albumin was low (29g/dl), and the retroviral screen was negative. Blood culture, gene Xpert, chest X-ray, D-dimer, genotype, and neck Doppler scan were requested, but they were not done due to financial constraints.
He was subsequently placed on weight-based low molecular weight heparin (LMWH) and subsequently bridged to warfarin tablets after a week of presentation, along with intravenous antibiotics (meropenem and vancomycin). Ocular care was provided with ofloxacin eye drops and assistance with toileting. Nutritional rehabilitation was commenced. Blood transfusion was performed for optimisation, and intensive physiotherapy was initiated. However, the patient was unable to consistently adhere to medications on several occasions due to financial constraints.
On the second week of admission, he was noticed to pass live worms per rectum, which were demonstrated to be Ascaris lumbricodes. Stool examination microscopy revealed ova of A. lumbricoides, while blood film for microfilaria was negative. He was given a stat dose of oral albendazole and weight-adjusted oral ivermectin by the medical parasitologist.
The patient made significant improvement with the resolution of fever, facial swelling, and ocular infection. He was discharged on the fourth week of admission to a follow-up clinic with a GCS of 15 with residual left hemiparesis.
We searched all published journals from inception until October 2023, on PUBMED, EMBASE, and GOOGLE SCHOLAR using the following search terms: “cerebral venous thrombosis” “dural sinus thrombosis” “helminthiasis,” and “worms.” Additionally, we also looked through the lists of references in relevant research papers to find any additional studies. We identified a total of seven case reports, of which five were males and two females, with a mean age of 38.4 years (range: 13–50 years).[5,6,7,8,9,10,11] All were adults except one.[8] Headache was the predominant symptom seen in all cases, with abducens nerve palsy reported in two cases. The superior sagittal sinus was the most common sinus involved (5/7). Mortality was only recorded in one of the cases[5] (see Table 1).
Table 1.
Reported cases of cerebral venous sinus thrombosis with associated helminthiasis
| Reference (Year of Publication) | Country | Specific Helminth | Clinical Features | Involved Sinus(es) | Treatment Modalities |
|---|---|---|---|---|---|
| Dalcin et al.[7] (2017) | Canada | Trichinella nativa | Headache, blurred vision, CN VI palsy, and bilateral papilledema | Superior sagittal sinus, left transverse sinus, and inferior and straight sinus | Corticosteroid, antihelminthics, anticoagulant |
| Barr[11] (1966) | Canada | Trichinella spiralis | Headache, fever, bilateral periorbital edema, and CN VI palsy | Cavernous sinus | Corticosteroid |
| Jia-Yan Wu et al.[6] (2013) | China | Paragonimus westermani | Loss of consciousness, seizure, and right hemiplegia | Superior sagittal sinus, sigmoid sinus, and left transverse sinus | Anticonvulsant, antihelminthic, anticoagulant |
| Evans et al.[10] (1982) | United States of America | Trichinella spiralis | Headache, vomiting, seizure, right hemiplegia, and papilledema | Superior saggital sinus | Corticosteroid, anticonvulsant |
| Gay et al.[5] (1982) | United States of America | Trichinella spiralis | Headache, photophobia, seizure, and left upper limb monoparesis | Multiple sinuses (not specified) | Corticosteroid |
| Prasad et al.[8] (2005) | Nepal | Taenia solium | Headache | Right lateral sinus | Antihelminthic |
| El Koussa et al.[9] (1994) | FTA | Trichinella spp | FTA | Superior sagittal sinus and left lateral sinus | FTA |
CN = cranial nerve, FTA = full text not available
Discussion
Fever, altered sensorium, and focal neurologic deficits are common clinical features of CVST, though not pathognomonic. Similar clinical patterns were noted in the reported cases (see Table 1). While helminthic infestation can hardly explain the fever, superimposed or concurrent bacterial infection may be responsible for the pyrexia. The leucocytosis with predominant neutrophilia in our patient supports this. Previous case reports (as shown in Table 1) had identified Trichinella as the most common worm associated with CVST. This is probably the first report of Ascaris in association with CVST. Poor sewage disposal and unsafe water consumption were likely the routes of helminthic exposure in our index case.
The consequences of helminthiasis on the central nervous system include both direct and indirect pathological effects, potentially leading to conditions such as epilepsy, encephalopathy, eosinophilic meningitis, and neurocysticercosis.[12] While establishing a true causal relationship between helminthiasis and CVST may be challenging, both conditions co-existed in our patient. Further studies are required to determine this association. Additionally, a noteworthy case involved the extraction of a live worm (Ophidoascaris robertsi) from the right frontal lobe of a 64-year-old immunocompromised female, underscoring the neurological significance of parasitic infestations.[13]
Tatter et al.[14] reported two cases of intestinal A. lumbricoides infestation, each with distinct cerebral manifestations. One patient had a 2 cm right mesial temporal lesion that subsequently resolved with antihelminthics, while the other developed a polymicrobial brain abscess, with A. lumbricodes hypothesised as a potential vector. Reports of CVST in the setting of helminthiasis appear to be limited in the literature, with a summary of documented cases tabulated [Table 1].
The proposed mechanisms of CVST in helminthiasis include hypereosinophilia, toxic vasculitis, and direct seeding within the sinus.[15] We hypothesised that A. lumbricoides may have played a synergistic role in the development of CVST in this index case via anaemia and chronic malnutrition resulting from loss of essential micro and macronutrients, with resultant immunosuppression.[16] Furthermore, anaemia has been recognised as an independent risk factor for cerebral venous thrombosis formation in paediatrics, particularly when it is chronic and due to iron deficiency.[17,18] Pointers to malnutrition in this case included low weight for age, low total serum albumin level, and hepatomegaly. The possible causes of CVST in our patient included anaemia, fever, a right facial boil with ocular infection, and helminthiasis.
The definitive diagnosis of helminthic invasion of the brain involves direct pathological demonstration of the ova, larva, or worms in the brain, which is invasive and not usually feasible. Thus, most diagnoses are made with supporting clinical, laboratory, and neuroimaging findings with clinical resolution at the commencement of antihelminthiasis. Magnetic resonance (MR) imaging with MR venography or computed tomogram (CT) scan with CT venography of the head and neck is recommended for diagnosis of CVST. In our sub-region, a cranial CT scan is usually used for diagnosis (either with or without contrast, as in this instance). This may lead to underreporting of cases, as CT scans without the complement of CT venography have lower sensitivity.[3,19,20]
Treatment is centred on antihelminthics and anticoagulants, addressing other underlying causes and controlling neurological complications such as seizures and raised intracranial pressure. While there is a paucity of well-designed randomised trials evaluating the effectiveness of anticoagulants in paediatrics, they should be started once the diagnosis is made and have been observed to be safe in this age group.[4,21] Our patient improved significantly with anticoagulants, antihelminthics, and other supportive care; however, he still has residual motor deficits. This has been noted to occur in patients who initially present with focal neurologic deficits, as in our index case.[22]
Poor healthcare financing, coupled with low socioeconomic status, continues to hamper optimum patient care. Our index patient was not fully compliant with his medications and requested investigations due to out-of-pocket health funding, emphasising the need for more inclusive healthcare insurance.
Conclusion
CVST, though uncommon, should be considered in patients presenting with altered sensorium, fever, and focal neurologic deficits, especially in the presence of intestinal A. lumbricoides. It is essential to establish investigation modalities in patients with CVST and helminthiasis to better understand the pathophysiology and determine its exact role, whether as a “direct causative agent” or a “potentiating factor.”
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
References
- 1.Ogunmodede AF, Sunmonu TA, Owhin SO, Owolabi GR, Komolafe MA. Challenges in the management of cerebral venous thrombosis in a federal medical center: A report of three cases. Ann Med Case Rep. 2022;4:1038. [Google Scholar]
- 2.Okunola PO, Ofovwe GE, Abiodun MT, Azunna CP. Superior sagittal sinus thrombosis complicating typhoid fever in a teenager. Case Rep Pediatr. 2012;2012:1–3. doi: 10.1155/2012/201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baduro Y, Ferro JM. Cerebral venous thrombosis in sub-Saharan Africa: A systematic review. J Stroke Cerebrovasc Dis. 2021;30:105712. doi: 10.1016/j.jstrokecerebrovasdis.2021.105712. [DOI] [PubMed] [Google Scholar]
- 4.Sébire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, et al. Cerebral venous sinus thrombosis in children: Risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477–89. doi: 10.1093/brain/awh412. [DOI] [PubMed] [Google Scholar]
- 5.Gay T, Pankey GA, Beckman EN, Washington P, Bell KA. Fatal CNS trichinosis. JAMA. 1982;247:1024–5. [PubMed] [Google Scholar]
- 6.Wu JY, Zhang BR, Zhao GH. Cerebral infarction and cranial venous sinus thrombosis caused by paragonimiasis. CNS Neurosci Ther. 2013;19:734–6. doi: 10.1111/cns.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalcin D, Zarlenga DS, Larter NC, Hoberg E, Boucher DA, Merrifield S, et al. Trichinella nativa outbreak with rare thrombotic complications associated with meat from a black bear hunted in Northern Ontario. Clin Infect Dis. 2017;64:1367–73. doi: 10.1093/cid/cix165. [DOI] [PubMed] [Google Scholar]
- 8.Prasad R, Singh R, Joshi B. Lateral sinus thrombosis in neurocysticercosis. Trop Doct. 2005;35:182–3. doi: 10.1258/0049475054620914. [DOI] [PubMed] [Google Scholar]
- 9.El Koussa S, Chemaly R, Fabre-Bou Abboud V, Tamraz J, Haddad N. Trichinose et occlusions sino-veineuses cerebrales. Rev Neurol (Paris) 1994;150:464–6. [PubMed] [Google Scholar]
- 10.Evans RW, Patten BM. Trichinosis associated with superior sagittal sinus thrombosis. Ann Neurol. 1982;11:216–7. doi: 10.1002/ana.410110225. [DOI] [PubMed] [Google Scholar]
- 11.Barr R. Human trichinosis: Report of four cases, with emphasis on central nervous system involvement, and a survey of 500 consecutive autopsies at the Ottawa Civic Hospital. Can Med Assoc J. 1966;95:912–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Katchanov J, Nawa Y. Helminthic invasion of the central nervous system: Many roads lead to Rome. Parasitol Int. 2010;59:491–6. doi: 10.1016/j.parint.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hossain ME, Kennedy KJ, Wilson HL, Spratt D, Koehler A, Gasser RB, et al. Human neural larva migrans caused by Ophidascaris robertsi Ascarid. Emerg Infect Dis. 2023;29:1900–3. doi: 10.3201/eid2909.230351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatter SB, Hopkins JW. Cerebral manifestations of Ascaris lumbricoides. Contemp Neurol. 1997;1997:253–2864. [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolić S, Vujosević M, Sasić M, Poluga J, Misić S, Najdanović L, et al. Neuroloske manifestacije u toku trihinoze [Neurologic manifestations in trichinosis] Srp Arh Celok Lek. 1998;126:209–13. [PubMed] [Google Scholar]
- 16.Nnolim C, Adekeye TA, Awobode HO. Intestinal helminth infection and malnutrition among public primary school children in Ibadan, Nigeria. Niger J Parasitol. 2020;41:49–55. [Google Scholar]
- 17.Shah P, Nguyen D, Berman B. Cerebral venous sinus thrombosis related to iron-deficiency anemia. Cureus. 2020;12:e8917. doi: 10.7759/cureus.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moiz B, Ukrani RD, Arif A, Akbar I, Sadiq MW, Altaf S. Case study of pediatric cerebral sinus venous thrombosis center of a low middle-income Country. Clin Appl Thromb. 2021;27:10760296211022847. doi: 10.1177/10760296211022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes C, Newall F, Furmedge J, Mackay M, Monagle P. Cerebral sinus venous thrombosis in children. J Paediatr Child Health. 2004;40:53–5. doi: 10.1111/j.1440-1754.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- 20.Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am. 2010;21:511–27. doi: 10.1016/j.nec.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stam J, de Bruijn SF, DeVeber G. Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002005. [DOI] [PubMed] [Google Scholar]
- 22.Korathanakhun P, Sathirapanya P, Geater SL, Petpichetchian W. Predictors of hospital outcome in patients with cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2014;23:2725–9. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.020. [DOI] [PubMed] [Google Scholar]