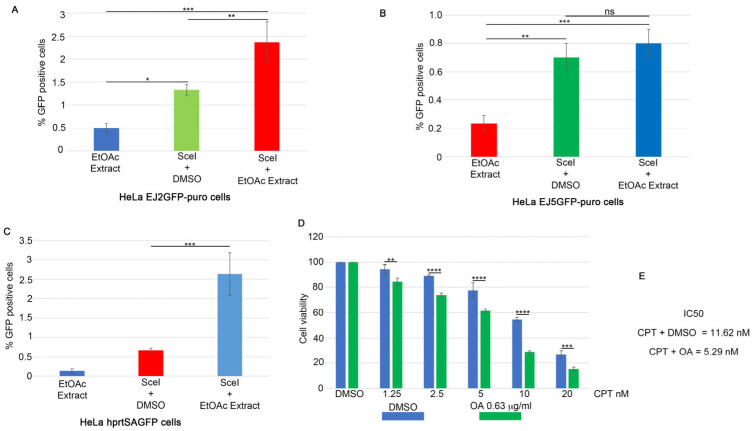
Figure 3.
OA reduces HeLa cell viability in combination with CPT. (A) HeLa cells stably expressing the EJ2GFP-puro reporter plasmid were transfected with the SceI plasmid, followed by incubation with EtOAc for 24 h. At the end of the incubation period, GFP levels were measured using FACS analysis to assess Alt-EJ frequency. Data are shown as means ± standard deviation from three independent experiments. Statistically significant differences are indicated by * p < 0.05, ** p < 0.01 and *** p < 0.001. (B) HeLa cells stably expressing the pimEJ5GFP reporter plasmid were transfected with the I-SceI coding plasmid, followed by incubation as described in (A). NHEJ activity was measured using FACS analysis, with GFP levels serving as an indicator of NHEJ frequency. Data are presented as means ± standard deviation from three independent experiments. Statistically significant differences are indicated by ** p < 0.01, *** p < 0.001. (C) HeLa cells carrying the SSA-GFP reporter plasmid were transfected with the I-SceI endonuclease and treated as described in (A). Statistically significant differences are indicated by *** p < 0.001. (D) OA at 0.63 μg/mL or DMSO was pre-incubated for one hour, followed by incubation with different concentrations of CPT for 72 h. The mean of three independent experiments is reported, followed by statistical analysis. ** p < 0.01, *** p < 0.001, **** p < 0.0001. (E) IC50 calculation, using GraphPad software, from cell viability assay of HeLa cells treated as described in (D).