Abstract
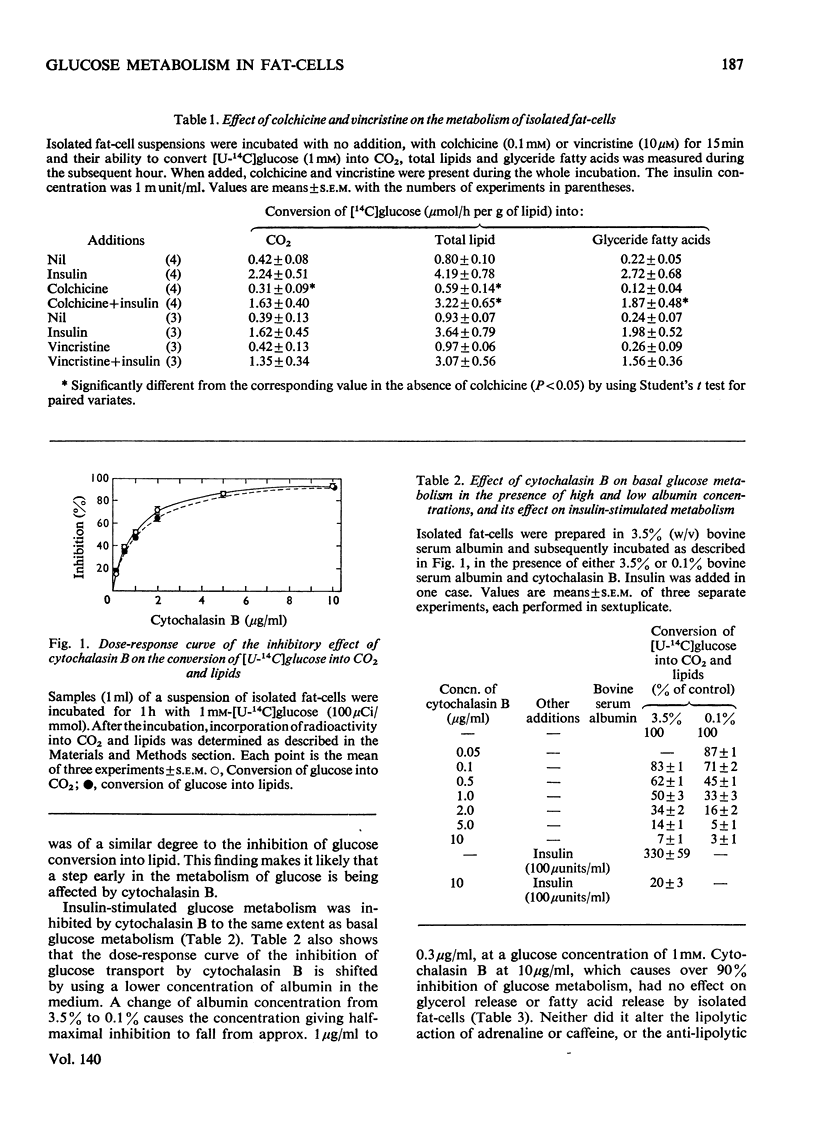
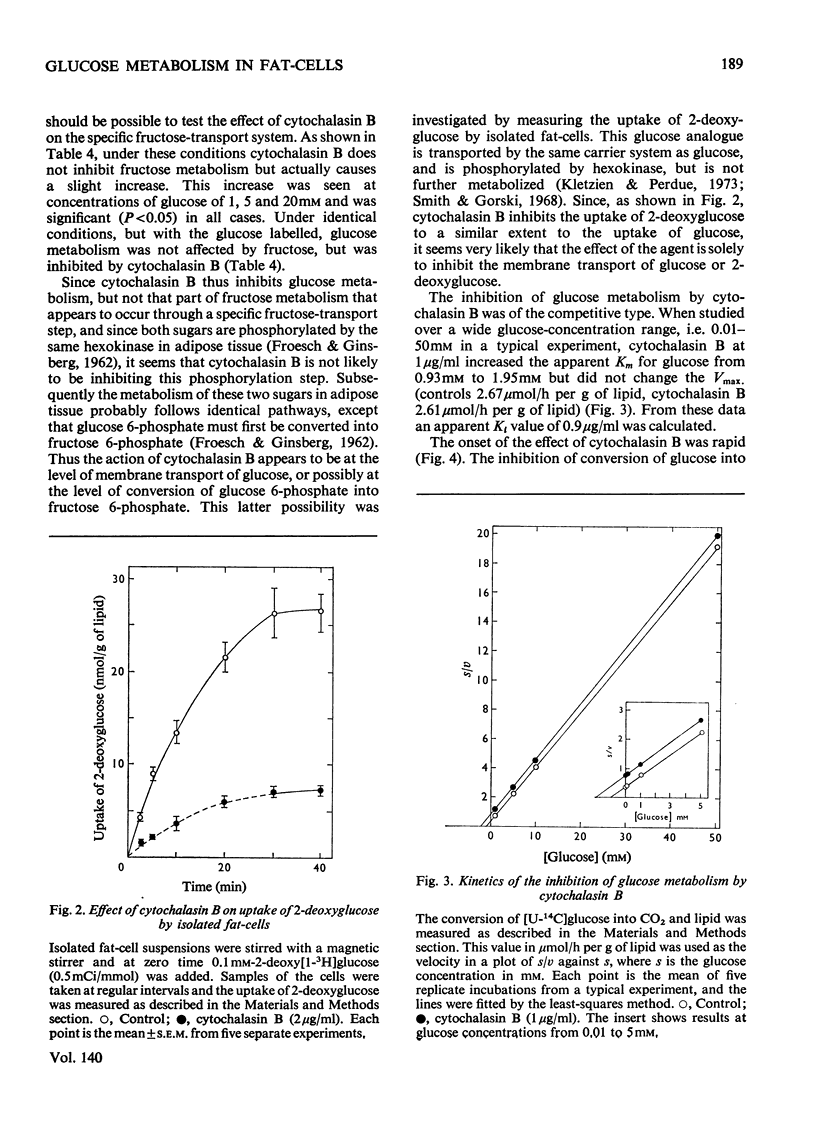
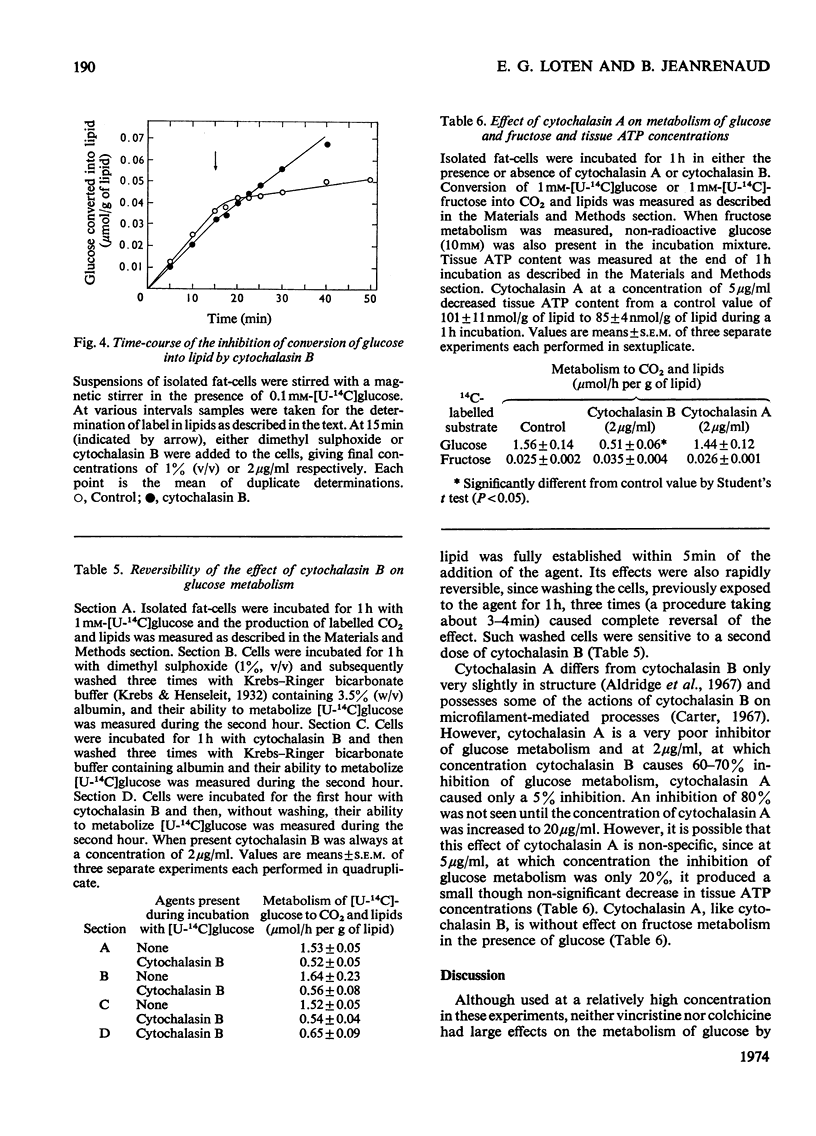
1. Colchicine and vincristine only slightly inhibit the metabolism of glucose to CO2 and lipids by isolated fat-cells. 2. Prolonged incubation with these agents causes no further inhibition. 3. Cytochalasin B, however, inhibits glucose metabolism to both CO2 and lipids in fat-cells. 4. However, at a concentration that causes a strong inhibition of glucose metabolism cytochalasin B is without effect on the metabolism of pyruvate, lactate or arginine to these end products. The uptake of labelled α-aminoisobutyrate is likewise not modified. Similarly it does not affect release of glycerol or free fatty acid, or the actions of adrenaline, insulin or caffeine on these parameters. At 10μg/ml it slightly lowers ATP concentrations, an effect that does not occur at 2μg/ml. 5. The transport of fructose into adipocytes by a specific fructose-transport system is also not affected by the agent, but the uptake of 2-deoxyglucose is strongly inhibited. It is concluded that cytochalasin B may specifically inhibit the glucose-transport system of isolated fat-cells. 6. Cytochalasin A has a much weaker action than cytochalasin B on glucose metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Butcher F. R., Goldman R. H. Effect of cytochalasin B and colchicine on the stimulation of -amylase release from rat parotid tissue slices. Biochem Biophys Res Commun. 1972 Jul 11;48(1):23–29. doi: 10.1016/0006-291x(72)90338-5. [DOI] [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. RATE-LIMITING STEPS AND SITE OF INSULIN ACTION. J Biol Chem. 1965 Jan;240:14–21. [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Crofford O. B. Countertransport of 3-O-methyl glucose in incubated rat epididymal adipose tissue. Am J Physiol. 1967 Jan;212(1):217–220. doi: 10.1152/ajplegacy.1967.212.1.217. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lynn D. G., Lynn W. S. Cytochalasin B-sensitive 2-deoxy-D-glucose transport in adipose cell ghosts. J Biol Chem. 1973 May 25;248(10):3636–3641. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- FROESCH E. R., GINSBERG J. L. Fructose metabolism of adipose tissue. I. Comparison of fructose and glucose metabolism in epididymal adipose tissue of normal rats. J Biol Chem. 1962 Nov;237:3317–3324. [PubMed] [Google Scholar]
- Gliemann J. Glucose metabolism and response to insulin of isolated fat cells and epididymal. fat pads. Acta Physiol Scand. 1968 Apr;72(4):481–491. doi: 10.1111/j.1748-1716.1968.tb03872.x. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Ho R. J. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem. 1970 Jul;36(1):105–113. doi: 10.1016/0003-2697(70)90337-4. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. The inhibition of sugar transport in chick embryo fibroblasts by cytochalasin B. Evidence for a membrane-specific effect. J Biol Chem. 1973 Jan 25;248(2):711–719. [PubMed] [Google Scholar]
- Malaisse W. J., Hager D. L., Orci L. The stimulus-secretion coupling of glucose-induced insulin release. IX. The participation of the beta cell web. Diabetes. 1972;21(2 Suppl):594–604. doi: 10.2337/diab.21.2.s594. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Walker M. O., Lacy P. E. The stimulus-secretion coupling of glucose-induced insulin release. V. The participation of a microtubular-microfilamentous system. Diabetes. 1971 May;20(5):257–265. doi: 10.2337/diab.20.5.257. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Inhibition of the transport of several hexoses in mammalian cells by cytochalasin B. J Biol Chem. 1972 Jun 25;247(12):4102–4105. [PubMed] [Google Scholar]
- Orci L., Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Rouiller C., Jeanrenaud B. Letter: Role of microtubules in lipoprotein secretion by the liver. Nature. 1973 Jul 6;244(5410):30–32. doi: 10.1038/244030a0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Smith D. E., Gorski J. Extrogen control of uterine glucose metabolism. An analysis based on the transport and phosphorylation of 2-deoxyglucose. J Biol Chem. 1968 Aug 25;243(16):4169–4174. [PubMed] [Google Scholar]
- Soifer D., Braun T., Hechter O. Insulin and microtubules in rat adipocytes. Science. 1971 Apr 16;172(3980):269–271. doi: 10.1126/science.172.3980.269. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Cytochalasin B inhibits thyroid secretion. Biochem Biophys Res Commun. 1971 Jul 16;44(2):422–425. doi: 10.1016/0006-291x(71)90617-6. [DOI] [PubMed] [Google Scholar]


