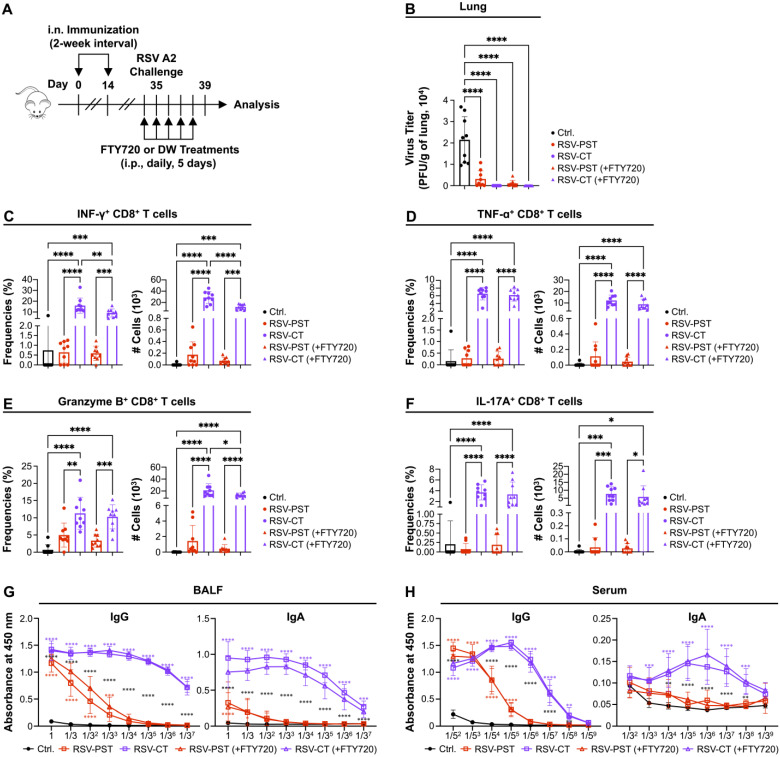
Figure 4.
Intranasal RSV-PST immunization provides mucosal protection against RSV A2 challenge by inducing systemic and local antigen-specific IgG production. BALB/c mice were immunized with intranasal administration of PBS, RSV-PST, or RSV-CT at 2-week intervals and challenged with RSV A2 strain (starting at day 35 post first immunization, intranasal route) in the presence or absence of FTY720 treatment (starting at day 34 post first immunization, i.p. route). The lung tissues were harvested and analyzed at 4 d.p.i. (A) Schematic of the experimental design. (B) Virus titers of RSV A2 strain within the lung tissues were measured by plaque assay. Frequencies and cell numbers of lung (C) IFN-γ+, (D) TNF-α+, (E) granzyme B+ (GrB), or (F) IL-17A+-activated CD8+ T cells that were re-stimulated with RSV-F protein for 18 h. The production levels of RSV-F-specific IgG and IgA in serial diluted (G) BALF and (H) serum were measured by ELISA. All data were pooled from two independent experiments (n = 4–5 per group). p values in (B–F) were calculated by ordinary one-way ANOVA. p values in (G,H) were analyzed by ordinary two-way ANOVA with Tukey’s multiple comparisons test (single pooled variance). A red or purple symbol * indicates a comparison to the control group. A black symbol * indicate a comparison between the RSV-PST and RSV-CT groups. Data are means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.