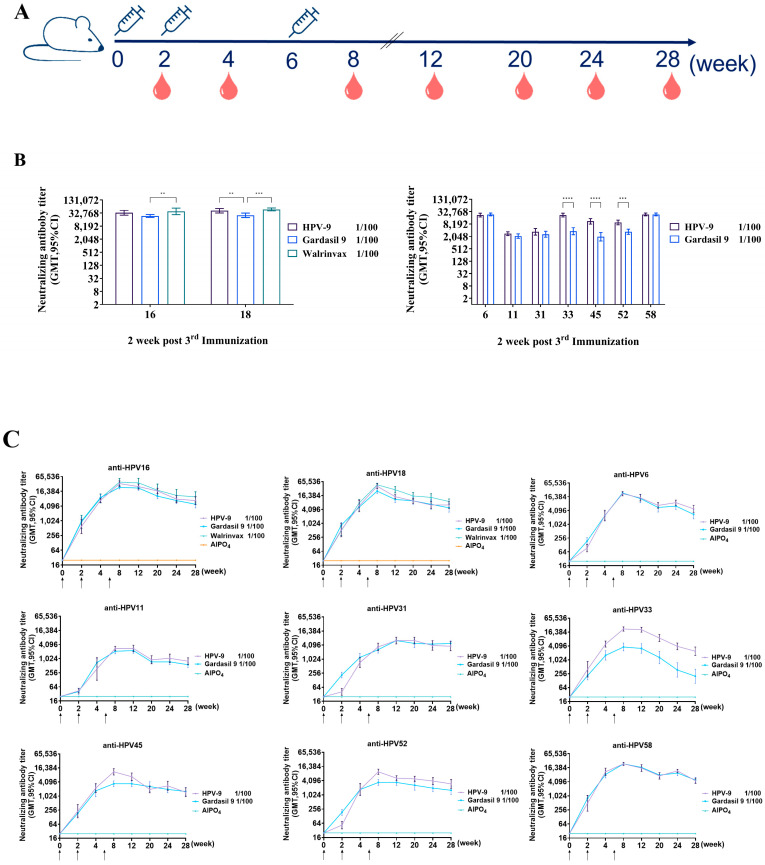
Figure 3.
The immunogenicity and immune persistence of the HPV-9 vaccine in BALB/c mice. (A) As the immunization routine scheme shows, female BALB/c mice were randomly divided into groups, with 10 mice per group. The mice were intraperitoneally injected with a 1/100 clinical dose of Gardasil® 9 and HPV-9 vaccine or Walrinvax® or the adjuvant as the placebo at weeks 0, 2, and 6; neutralizing assays were conducted at weeks 2, 4, 8, 12, 20, 24 and 28. (B) Two weeks after the 3rd vaccination, the neutralizing antibody titers tested through neutralizing experiments demonstrated that the HPV-9 vaccine’s effectiveness is comparable to that of Gardasil® 9 and Walrinvax® (p ≤ 0.01 (**), ≤0.001 (***), and <0.0001 (****)). (C) To evaluate the long-term protection induced by the HPV-9 vaccine in comparison with Gardasil® 9 or Walrinvax®, neutralizing assays were conducted to test the antibody-specific neutralizing antibodies in serum from vaccinated mice. The arrowheads represent the immunization timepoints.