Abstract
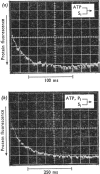
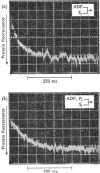
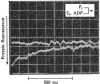
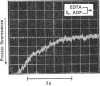
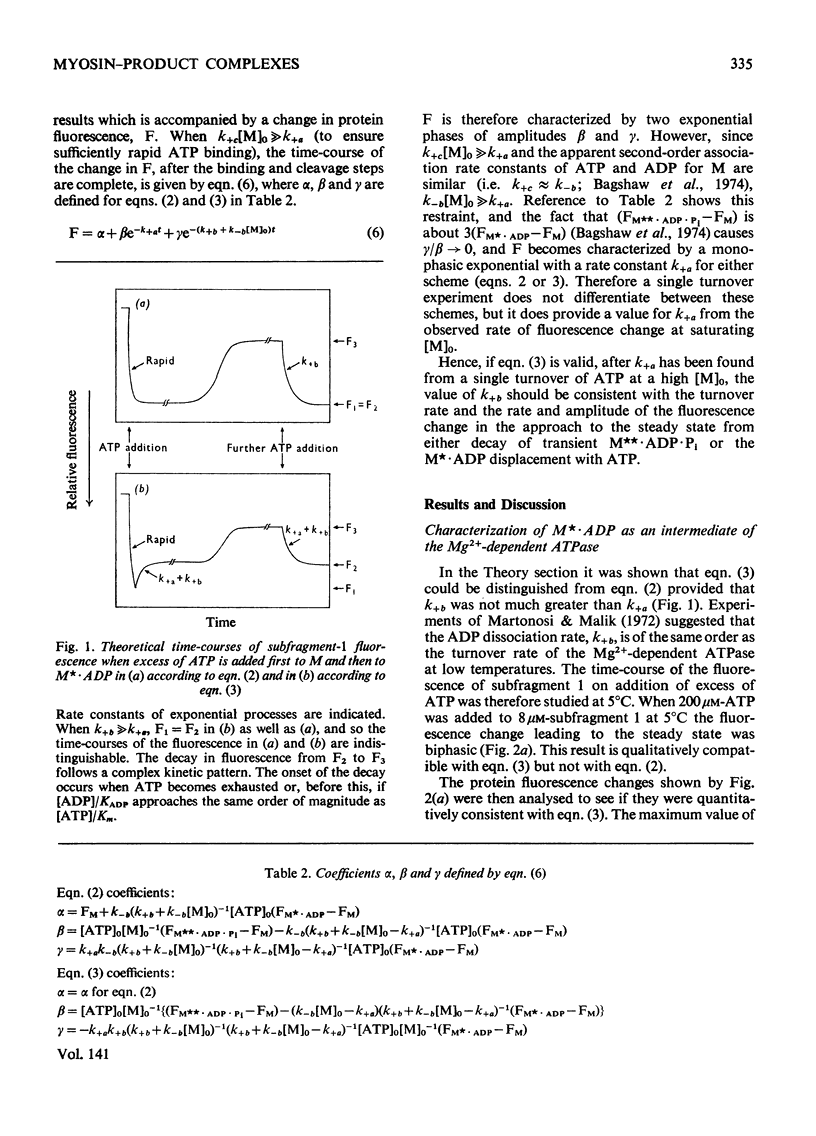
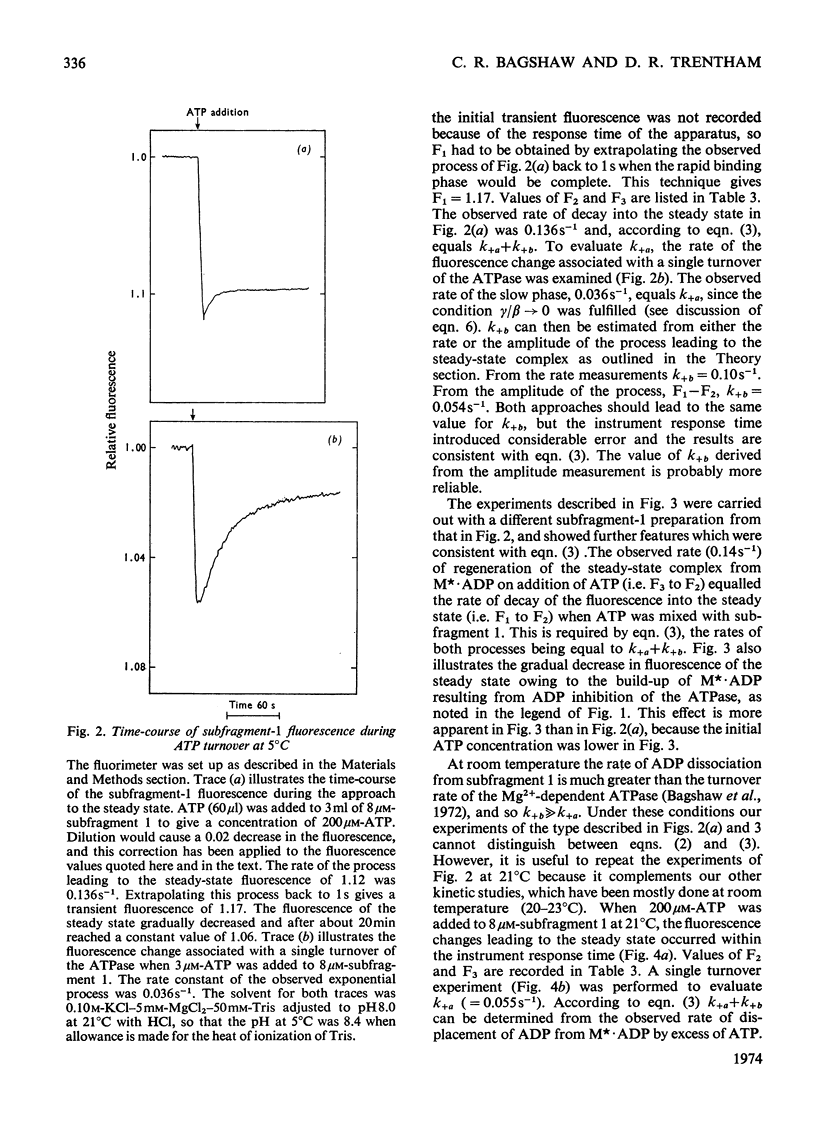
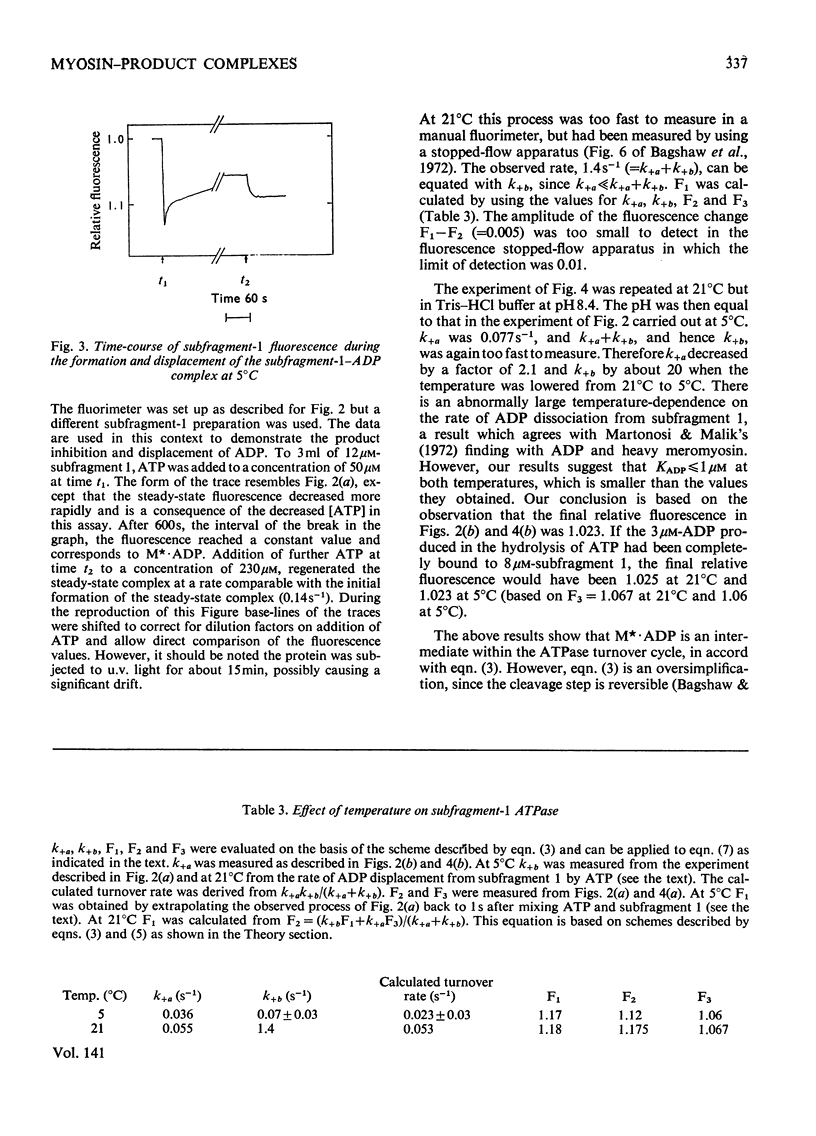
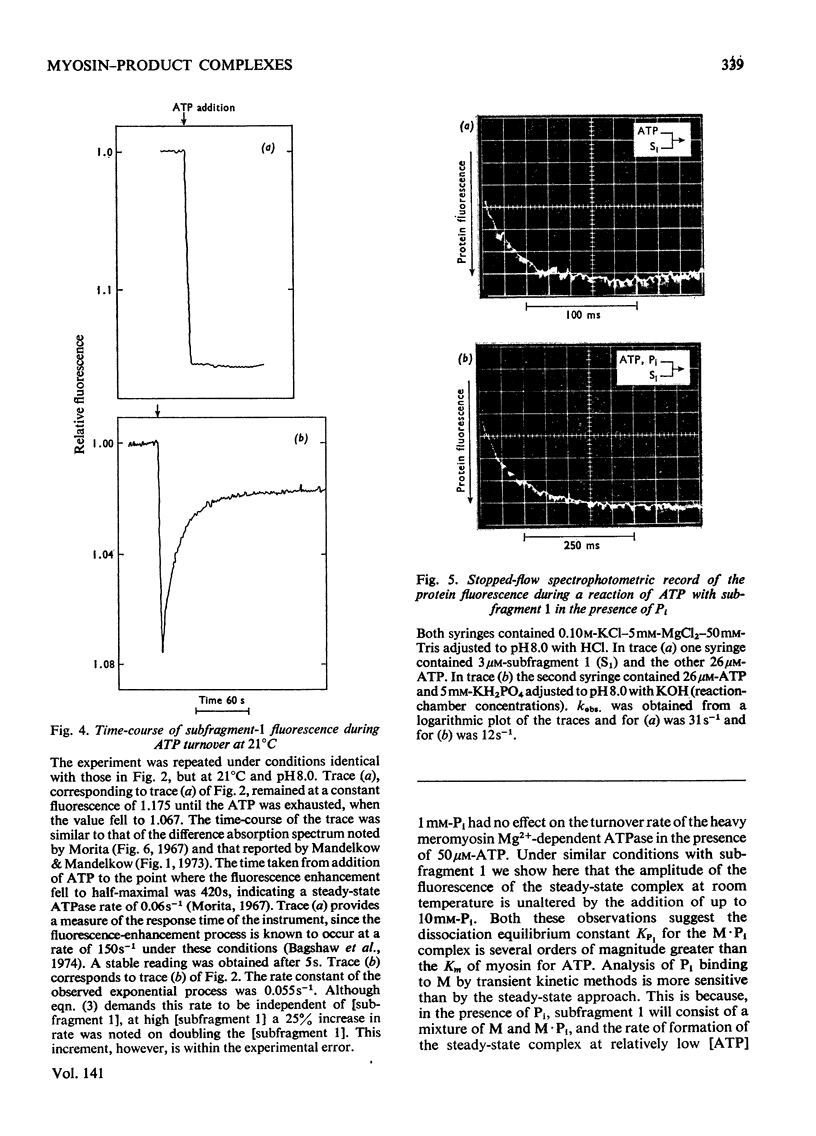
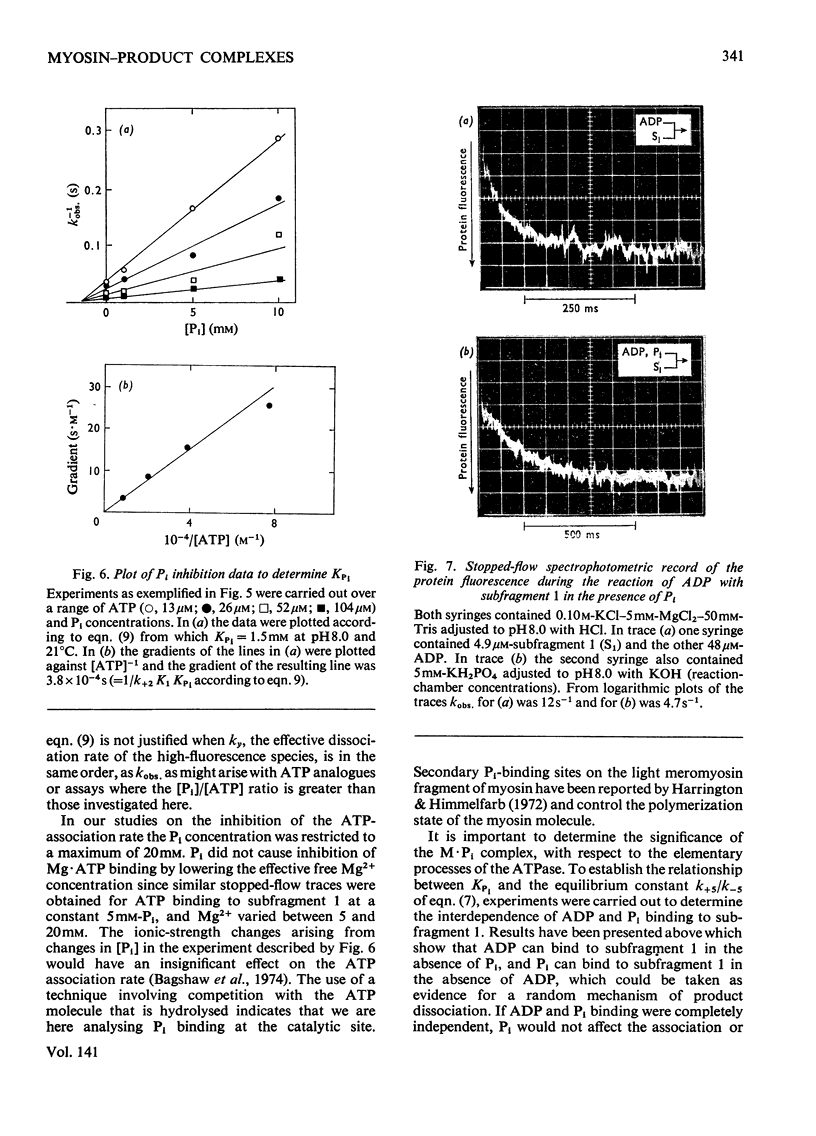
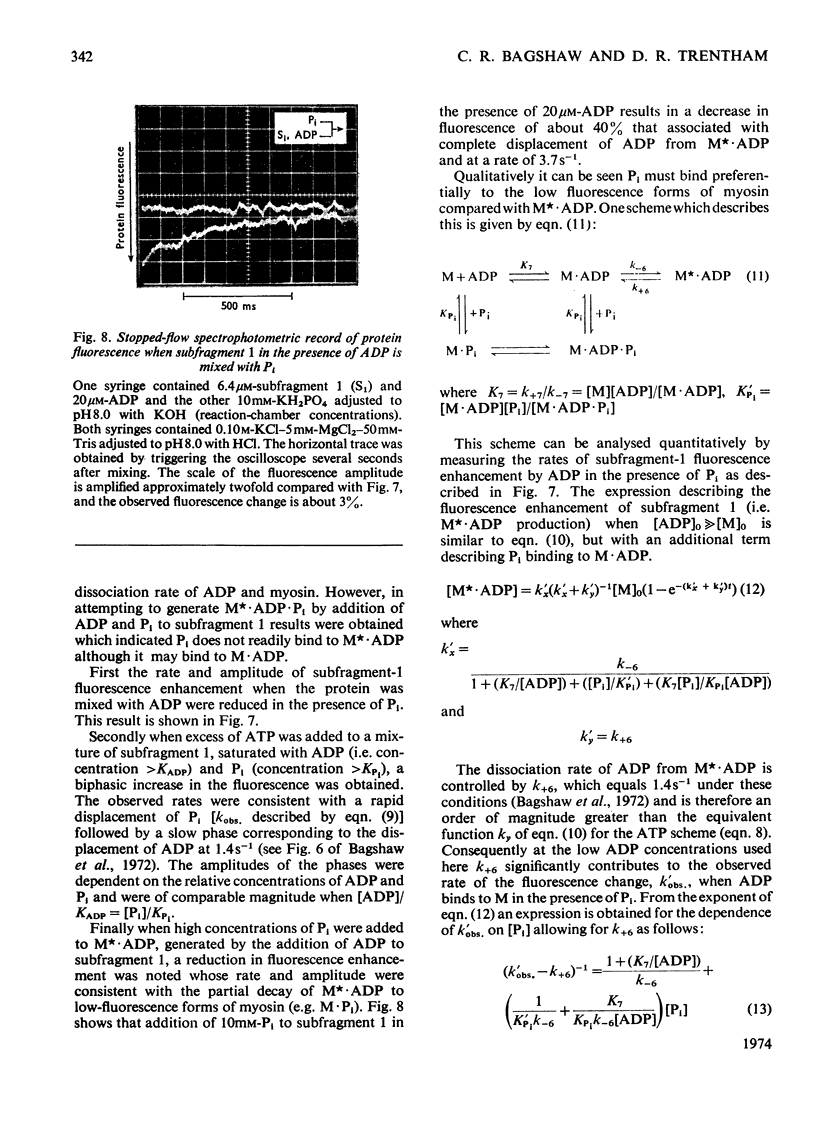
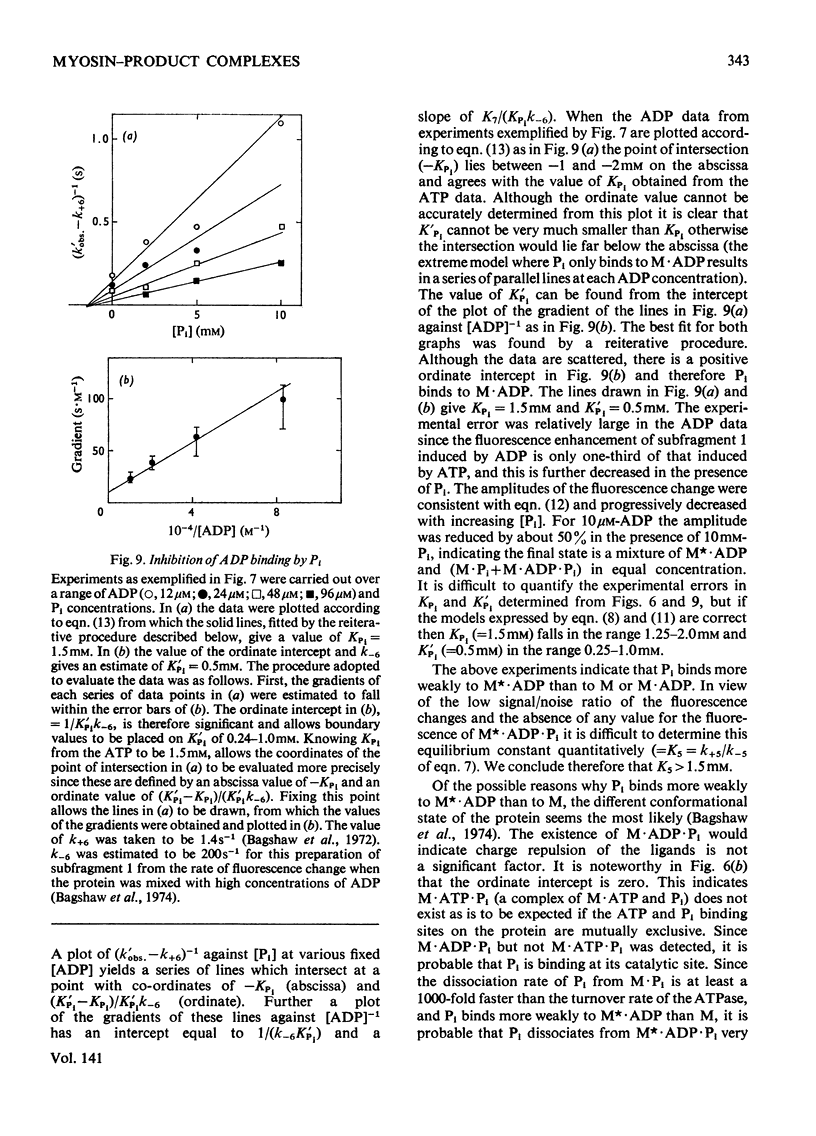
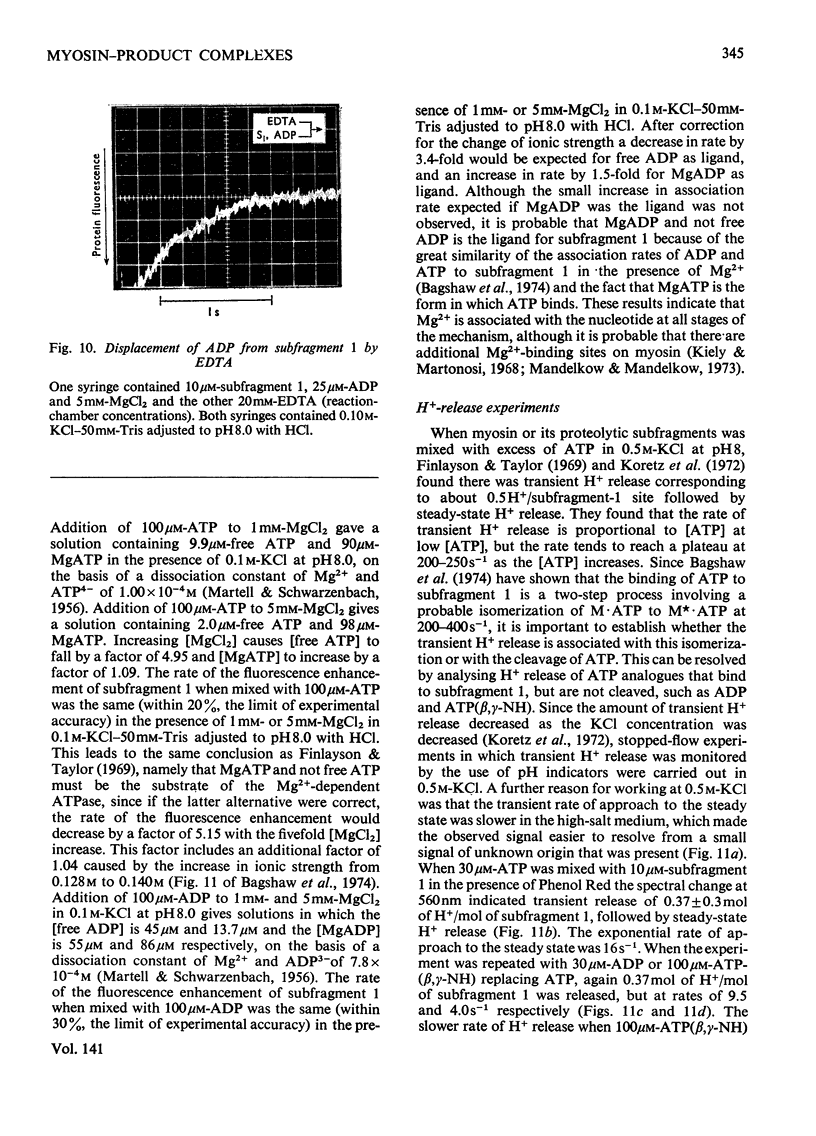
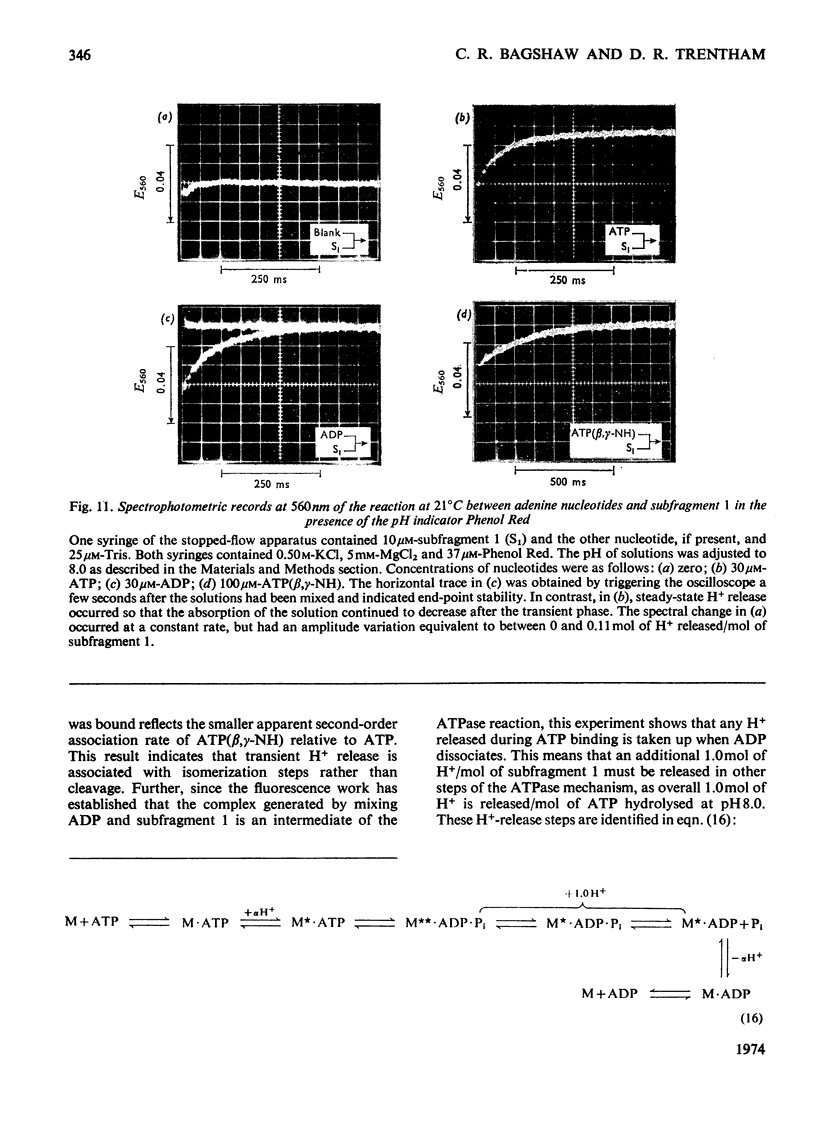
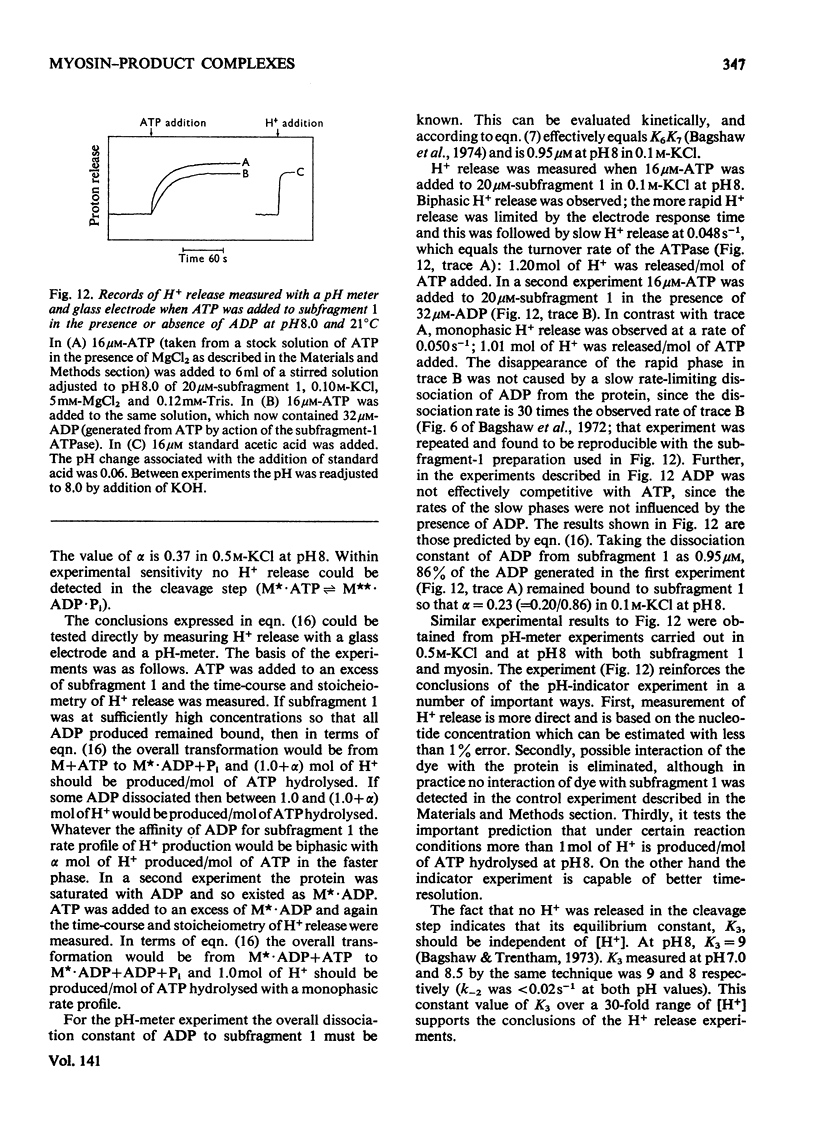
Evidence is presented that the myosin subfragment-1–ADP complex, generated by the addition of Mg2+ and ADP to subfragment 1, is an intermediate within the myosin Mg2+-dependent adenosine triphosphatase (ATPase) turnover cycle. The existence of this species as a steady-state intermediate at pH8 and 5°C is demonstrated by fluorescence measurements, but its concentration becomes too low to measure at 21°C. This arises because there is a marked temperature-dependence on the rate of the process controlling ADP dissociation from subfragment 1 (rate=1.4s−1 at 21°C, 0.07s−1 at 5°C). In the ATPase pathway this reaction is in series with a relatively temperature-insensitive process, namely an isomerization of the subfragment-1–product complex (rate=0.055s−1 at 21°C, 0.036s−1 at 5°C). By means of studies on the Pi inhibition of nucleotide-association rates, a myosin subfragment-1–Pi complex was characterized with a dissociation equilibrium constant of 1.5mm. Pi appears to bind more weakly to the myosin subfragment-1–ADP complex. The studies indicate that Pi dissociates from subfragment 1 at a rate greater than 40s−1, and substantiates the existence of a myosin-product isomerization before product release in the elementary processes of the Mg2+-dependent ATPase. In this ATPase mechanism Mg2+ associates as a complex with ATP and is released as a complex with ADP. In 0.1m-KCl at pH8 1.0mol of H+ is released/mol of subfragment 1 concomitant with the myosin-product isomerization or Pi dissociation, and 0.23 mol of H+ is released/mol of subfragment when ATP binds to the protein, but 0.23 mol of H+ is taken up again from the medium when ADP dissociates. Within experimental sensitivity no H+ is released into the medium in the step involving ATP cleavage.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Eccleston J. F., Eckstein F., Goody R. S., Gutfreund H., Trentham D. R. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J. 1974 Aug;141(2):351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch W. W., Moos C. Effect of temperature on actin activation of heavy meromyosin ATPase. Biochim Biophys Acta. 1971 May 11;234(2):183–189. doi: 10.1016/0005-2728(71)90073-9. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson B., Taylor E. W. Hydrolysis of nucleoside triphosphates by myosin during the transient state. Biochemistry. 1969 Mar;8(3):802–810. doi: 10.1021/bi00831a007. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. II. Evidence for the presence of a monomer--dimer equilibrium. Biochemistry. 1970 Feb 17;9(4):894–908. doi: 10.1021/bi00806a026. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Himmelfarb S. Effect of adenosine di- and triphosphates on the stability of synthetic myosin filaments. Biochemistry. 1972 Aug 1;11(16):2945–2952. doi: 10.1021/bi00766a004. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J. Protein fluorescence of lactate dehydrogenase. Biochem J. 1972 Jul;128(4):921–931. doi: 10.1042/bj1280921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui-Bon-Hoa G., Douzou P. Ionic strength and protonic activity of supercooled solutions used in experiments with enzyme systems. J Biol Chem. 1973 Jul 10;248(13):4649–4654. [PubMed] [Google Scholar]
- Kiely B., Martonosi A. Kinetics and substrate binding of myosin adenosine triphosphatase. J Biol Chem. 1968 May 10;243(9):2273–2278. [PubMed] [Google Scholar]
- LEVY H. M., SHARON N., RYAN E. M., KOSHLAND D. E., Jr Effect of temperature on the rate of hydrolysis of adenosine triphosphate and inosine triphosphate by myosin with and without modifiers. Evidence for a change in protein conformation. Biochim Biophys Acta. 1962 Jan 1;56:118–126. doi: 10.1016/0006-3002(62)90532-2. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Transient state phosphate production in the hydrolysis of nucleoside triphosphates by myosin. Biochemistry. 1970 Jul 21;9(15):2975–2983. doi: 10.1021/bi00817a007. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Fluorimetric studies on the influence of metal ions and chelators on the interaction between myosin and ATP. FEBS Lett. 1973 Jul 1;33(2):161–166. doi: 10.1016/0014-5793(73)80183-8. [DOI] [PubMed] [Google Scholar]
- Morita F. Interaction of heavy meromyosin with substrate. I. Difference in ultraviolet absorption spectrum between heavy meromyosin and its Michaelis-Menten complex. J Biol Chem. 1967 Oct 10;242(19):4501–4506. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Sartorelli L., Fromm H. J., Benson R. W., Boyer P. D. Direct and 18-O-exchange measurements relevant to possible activated or phosphorylated states of myosin. Biochemistry. 1966 Sep;5(9):2877–2884. doi: 10.1021/bi00873a015. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Watterson J. G. Conformational differences in the myosin-ADP complex in myofibrils and isolated myosin. FEBS Lett. 1973 Mar 15;30(3):305–308. doi: 10.1016/0014-5793(73)80675-1. [DOI] [PubMed] [Google Scholar]
- Talsky G. The anomalous temperature dependence of enzyme-catatlyzed reactions. Angew Chem Int Ed Engl. 1971 Aug;10(8):548–554. doi: 10.1002/anie.197105481. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viniegra-Gonzalez G., Morales M. F. Toward a theory of muscle contraction. J Bioenerg. 1972 May;3(1):55–64. doi: 10.1007/BF01515997. [DOI] [PubMed] [Google Scholar]
- Werber M. M., Szent-Györgyi A. G., Fasman G. D. Fluorescence studies on heavy meromyosin-substrate interaction. Biochemistry. 1972 Jul 18;11(15):2872–2883. doi: 10.1021/bi00765a021. [DOI] [PubMed] [Google Scholar]
- Yamada T., Shimizu H., Suga H. A kinetic study of the energy storing enzyme-product complex in the hydrolysis of ATP by heavy meromyosin. Biochim Biophys Acta. 1973 Jun 28;305(3):642–653. doi: 10.1016/0005-2728(73)90083-2. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]