Abstract
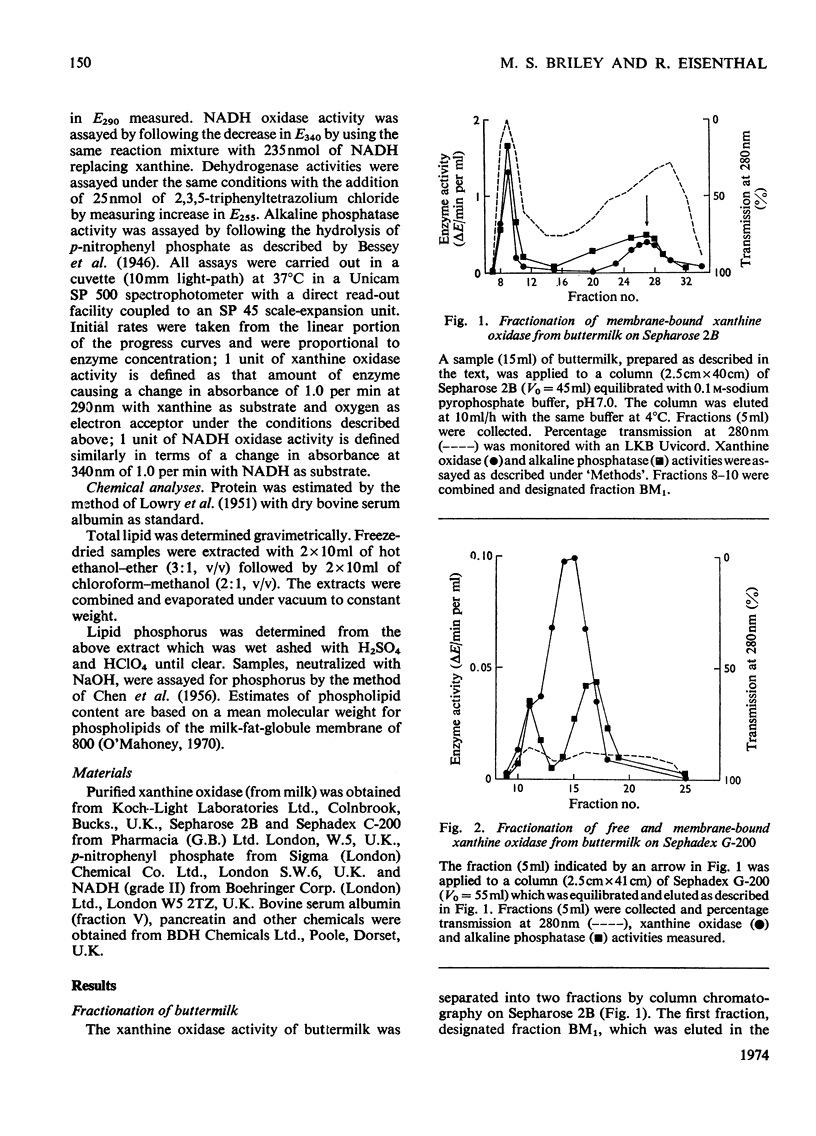
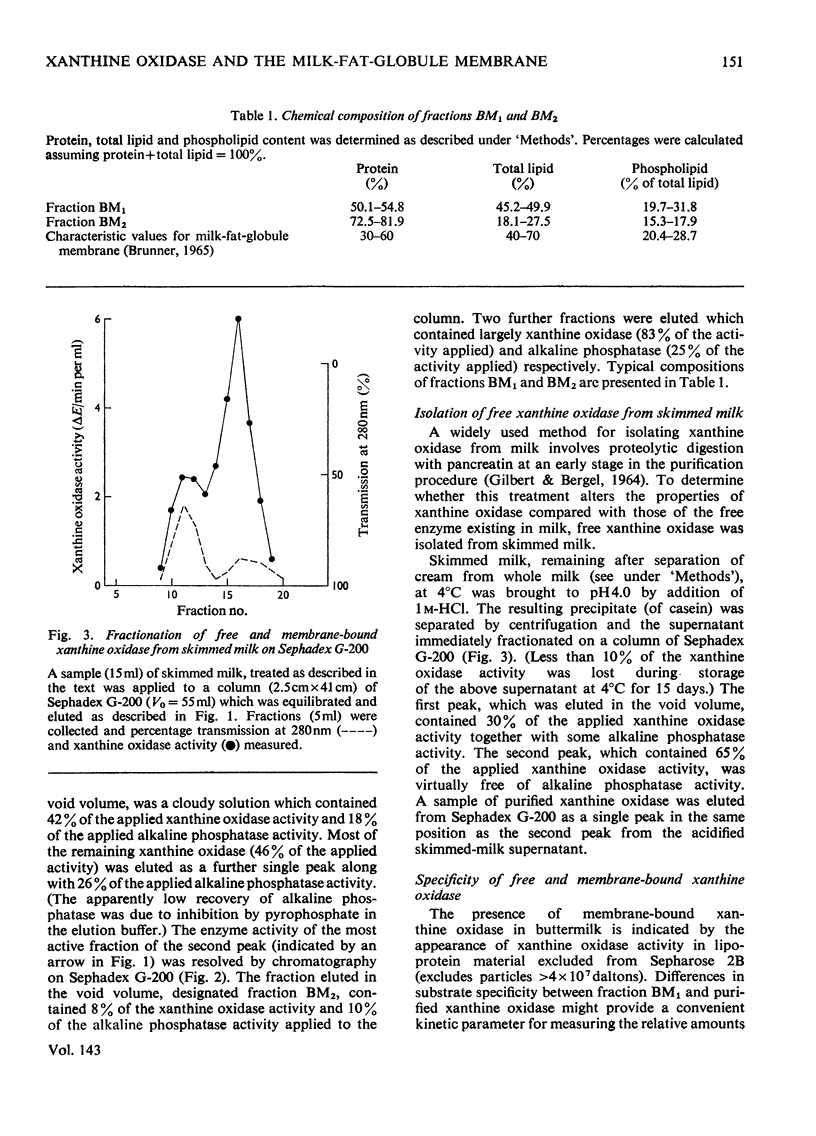
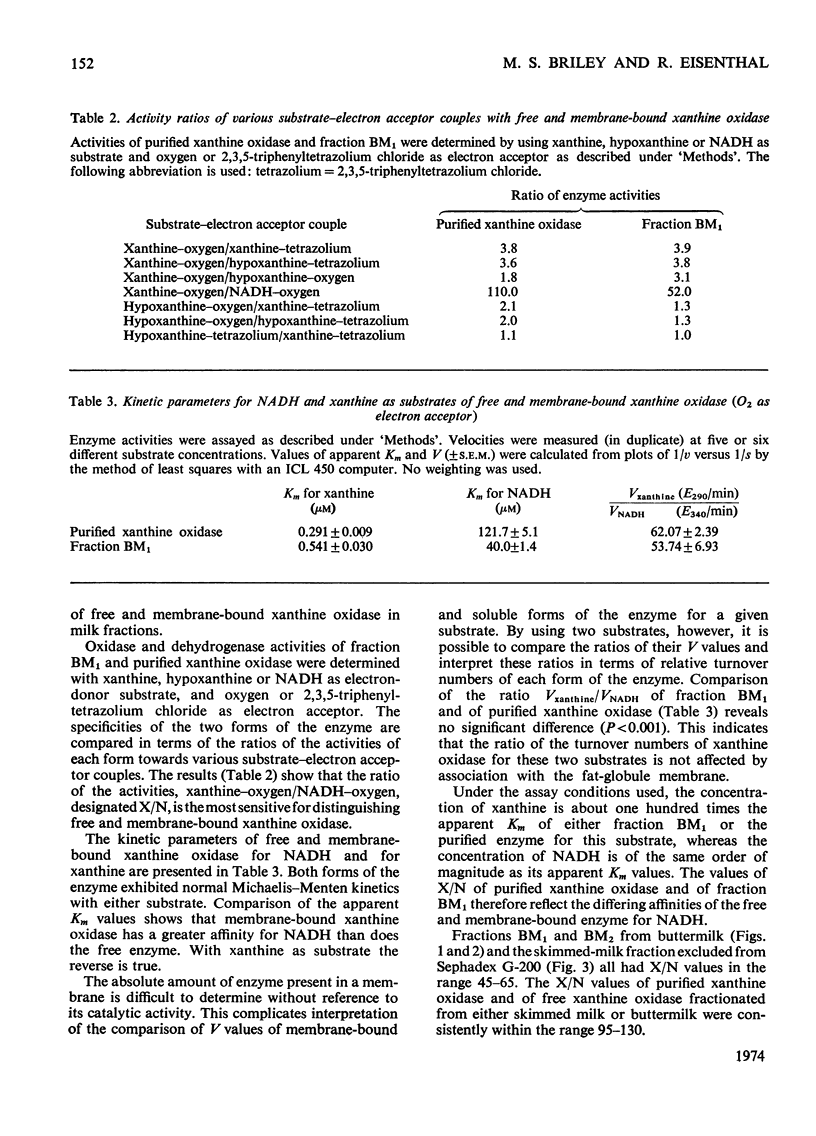
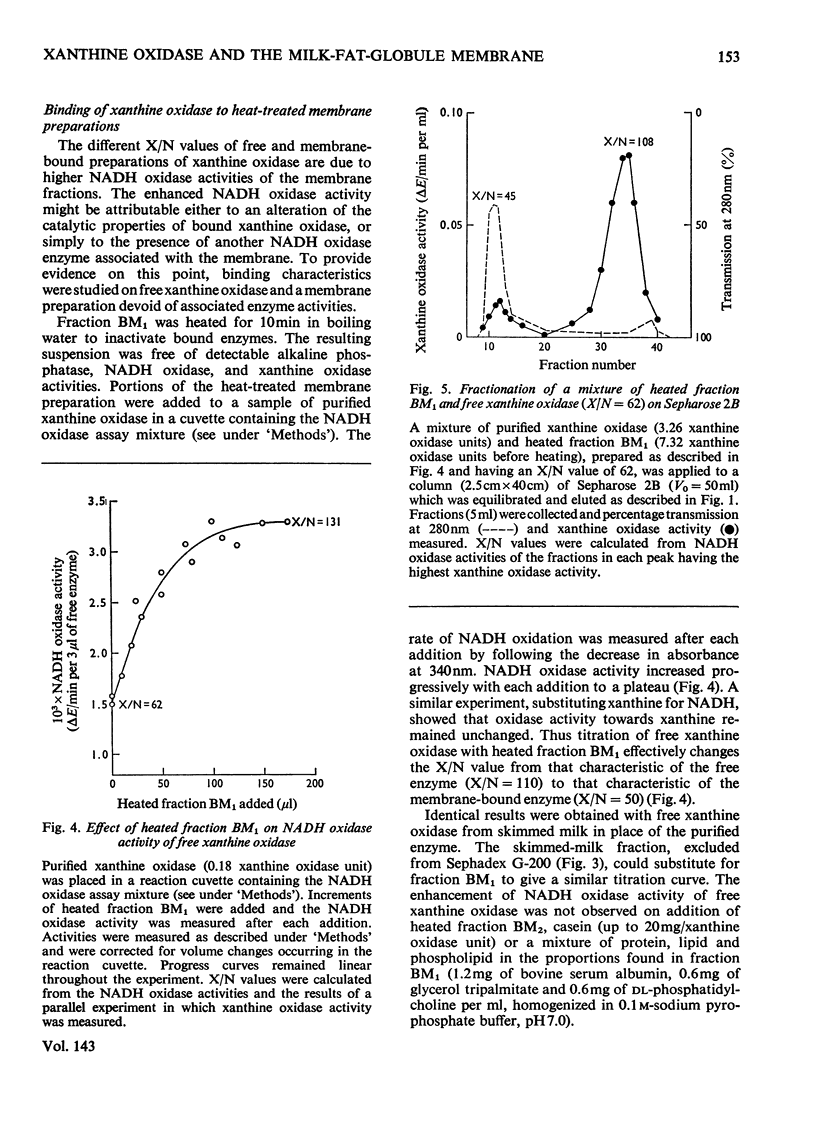
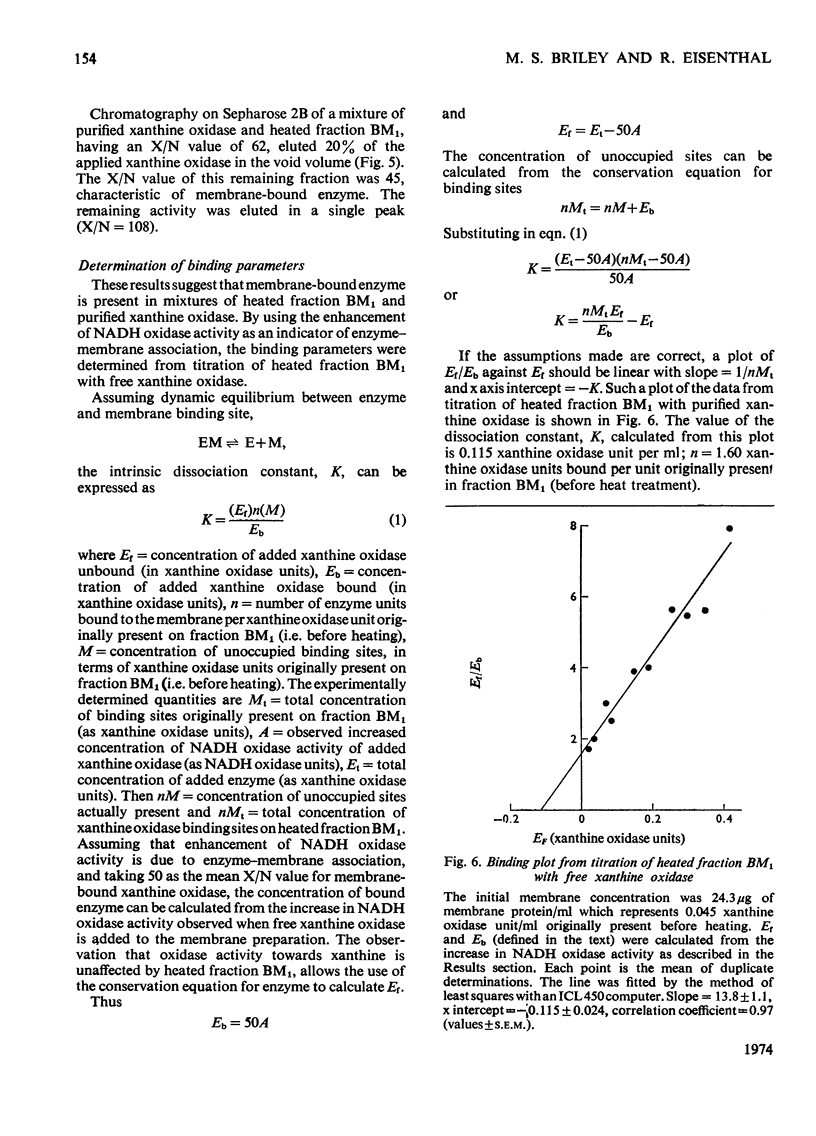
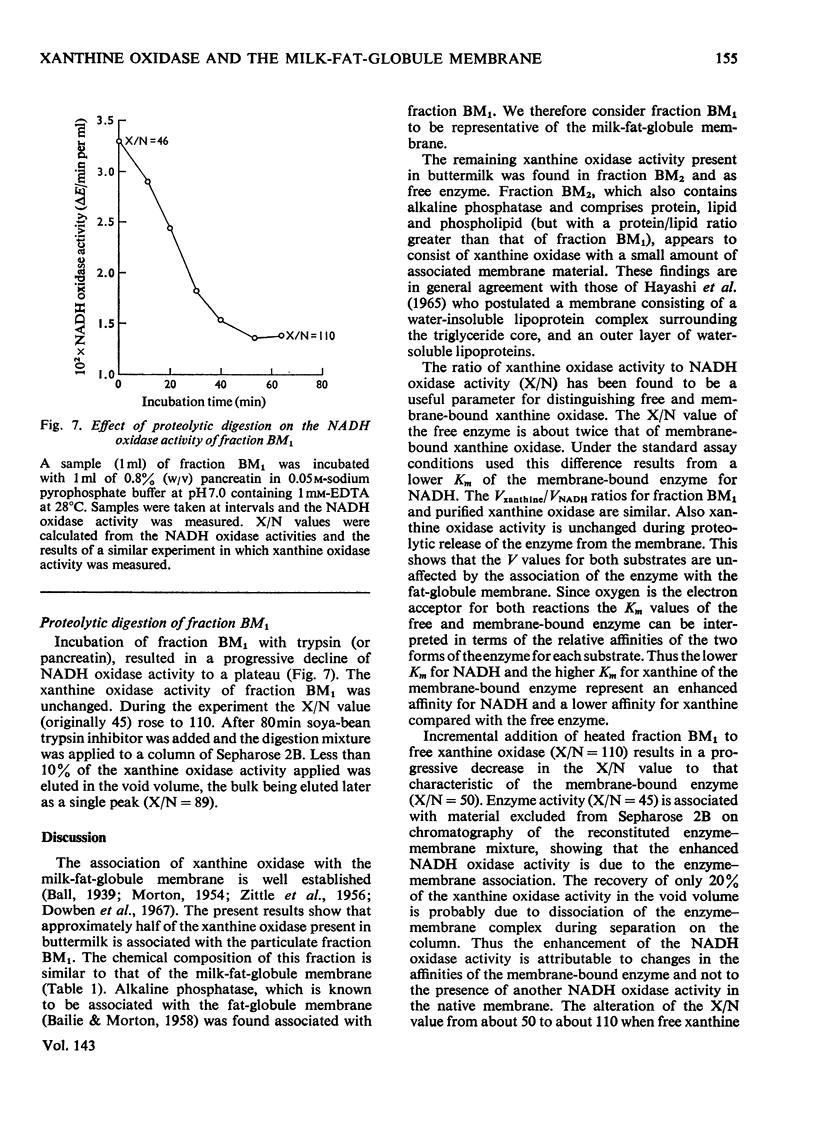
1. The catalytic properties of xanthine oxidase in bovine milk (EC 1.2.3.2) are dependent on the state of the enzyme, i.e. whether free or bound to the fat-globule membrane. Oxidase activity of the membrane-bound enzyme towards NADH is enhanced relative to that towards xanthine. This reflects a change in the relative Km values and enables the ratio of xanthine to NADH oxidase activities (X/N) to be used as a parameter for the relative amounts of free and membrane-bound xanthine oxidase in milk fractions. 2. Chromatography of buttermilk on Sepharose 2B yielded an excluded fraction, BM1, with xanthine oxidase activity. The remaining xanthine oxidase activity was eluted as a single broad peak. This was further resolved on Sephadex G-200 into an excluded fraction, BM2, and free xanthine oxidase. Fractions BM1 and BM2 had X/N values in the range 45–65, which is characteristic of membrane-bound xanthine oxidase. Purified xanthine oxidase has a mean X/N value of 110.3. Addition of fraction BM1, heated to remove associated enzyme activities, to purified xanthine oxidase progressively enhanced its NADH oxidase activity to a value where its X/N value was characteristic of membrane-bound xanthine oxidase. This was shown to be due to binding of free enzyme to heated fraction BM1. The binding constant and stoicheiometry were determined. 4. Proteolytic digestion of fraction BM1 liberated free xanthine oxidase from the fat-globule membrane with a corresponding alteration in X/N value.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P., Bray R. C., Edwards P., Shooter K. V. The chemistry of xanthine oxidase. 11. Ultracentrifuge and gel-filtration studies on the milk enzyme. Biochem J. 1964 Dec;93(3):627–632. doi: 10.1042/bj0930627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILIE M. J., MORTON R. K. Comparative properties of microsomes from cow's milk and from mammary gland. 1. Enzymic activities. Biochem J. 1958 May;69(1):35–44. doi: 10.1042/bj0690035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY F. G., FRIDOVICH I., HANDLER P. Preparation of several forms of xanthine oxidase by enzymic proteolysis. Biochim Biophys Acta. 1961 Oct 28;53:440–442. doi: 10.1016/0006-3002(61)90468-1. [DOI] [PubMed] [Google Scholar]
- Corran H. S., Dewan J. G., Gordon A. H., Green D. E. Xanthine oxidase and milk flavoprotein: With an Addendum by J. St L. Philpot. Biochem J. 1939 Oct;33(10):1694–1708. doi: 10.1042/bj0331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowben R. M., Brunner J. R., Philpott D. E. Studies on milk fat globule membranes. Biochim Biophys Acta. 1967 Feb 1;135(1):1–10. doi: 10.1016/0005-2736(67)90002-8. [DOI] [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Gilbert D. A., Bergel F. The chemistry of xanthine oxidase. 9. An improved method of preparing the bovine milk enzyme. Biochem J. 1964 Feb;90(2):350–353. doi: 10.1042/bj0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Erickson D. R., Smith L. M. Membranous material of bovine milk fat globules. II. Some physical and enzymic properties of the deoxycholate-released lipoproteins. Biochemistry. 1965 Dec;4(12):2557–2565. doi: 10.1021/bi00888a003. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Olson D. E., Mollenhauer H. H. Origin of the milk fat globule membrane. J Dairy Sci. 1971 Mar;54(3):295–299. doi: 10.3168/jds.S0022-0302(71)85832-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACKLER B., MAHLER H. R., GREEN D. E. Studies on metalloflavoproteins. I. Xanthine oxidase, a molybdoflavoprotein. J Biol Chem. 1954 Sep;210(1):149–164. [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- Nelson C. A., Handler P. Preparation of bovine xanthine oxidase and the subunit structures of some iron flavoproteins. J Biol Chem. 1968 Oct 25;243(20):5368–5373. [PubMed] [Google Scholar]


