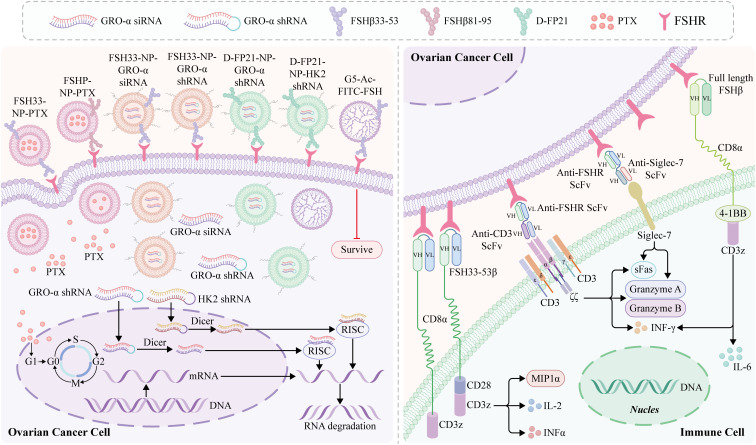
Figure 3.
Nanotechnology and immunotherapy for ovarian cancer (OC) targeting FSHR. Follicle-stimulating hormone peptide (FSHP) facilitates paclitaxel nanoparticles’ (NPs) targeting of ovarian carcinoma in vivo (14). The FSHP-NP-PTX system recognizes metastasic lymph nodes of ovarian cancer and can be captured by the lymph nodes by FSHP-NP-PTX passively targeting the lymphatic system, thereby significantly inhibiting cell proliferation (105). An siRNA-targeted NP delivery system with follicle-stimulating hormone (FSH)β 33-53 peptide as the targeting ligand inhibits the migration and invasion of ovarian clear cell cancer cells, which is an effective targeted therapy strategy for ovarian cancer and a stable delivery system for siRNA (106). FSH peptide-conjugated NPs with an increased amount of polyethylene glycol (PEG) grafting and encapsulated short hairpin RNA (shRNA) can silence the FSH target gene, growth-regulated oncogene α (gro-α) (107). Moli et al. (108) designed a novel FSH 33-targeting dendritic macromolecular nanocarrier as a potential delivery platform for OC cells that express FSHR, which is a highly effective active targeting medium and has the potential to block the FSH signaling pathway cascade while selectively delivering chemotherapy drugs, potentially enhancing its therapeutic effect. Preparation of ovarian cancer FSHR NP vector carrying therapeutic plasmid growth-regulating oncogene α (pGRO-a) short hairpin RNA (shRNA) (FP21-PEG-PEI/pGRO-a). A strategy to treat human ovarian cancer by redirecting primary human T cells to target FSHR laying a foundation for further development of FSHR-targeted immunotherapy (109). In addition, T-cells redirected against FSHR+ tumor cells with full-length FSH represent a promising therapeutic alternative against a broad range of ovarian malignancies, with negligible toxicity even in the presence of cognate targets in tumor-free ovaries (89). mAb targeting the external domain of FSHR using an in vivo-expressed FSHR vector and identifying an effective surface targeting mAb D2 AP 11 (10). DB7.2xD2AP11 DNA-encoded bispecific NK cell engager exhibits in vitro expression and binding to Siglec-7 and FSHR, which induces potent killing in multiple ovarian tumor lines and decreases tumor burden in vivo (110).