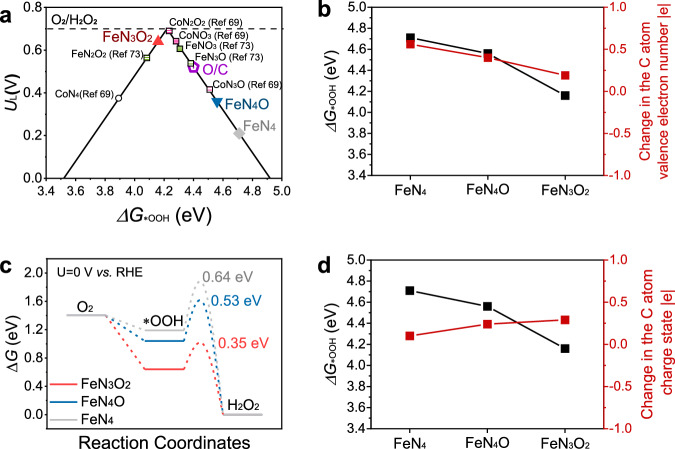
Fig. 6. Elucidation of the mechanism of efficient ORR catalysis by the FeN5−xOx sites for H2O2 production.
a Volcano plots of the computed activity of electrochemical H2O2 production via the 2e− ORR. The horizontal dash line indicates the equilibrium potential of O2/H2O2. Except for the FeN3O2, FeN4O, FeN4, and O/C sites, the sites labelled in black were adapted from the references. b Calculated *OOH adsorption energies and charges of the valence electron number of the C atom in the FeN3O2, FeN4O, and FeN4 sites. c Free energy diagram for the 2e− ORR of the FeN3O2, FeN4O, and FeN4 sites. The annotation values represent the computed kinetic barriers for *OOH to H2O2. d Calculated *OOH adsorption energies and changes in the C atom charge state at the FeN3O2, FeN4O, and FeN4 sites.