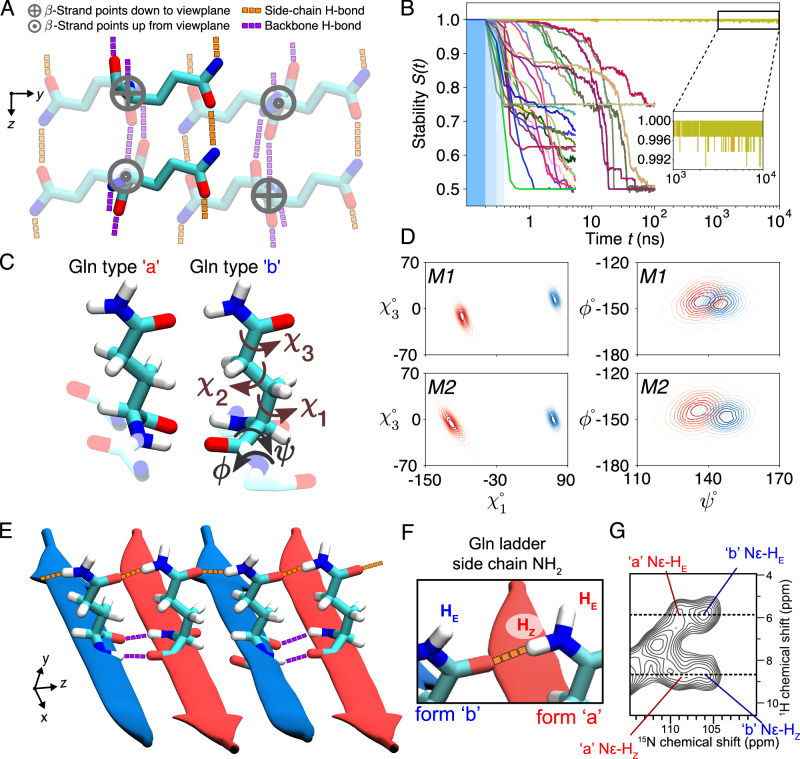
Fig. 2. Glutamine zippers and ladders within the polyQ amyloid core.
A The eight-Gln building block used to generate polyQ core candidates. Six residues are shown faded out to bring the two non-faded in focus: these two are on adjacent β-strands of an antiparallel β-sheet, stabilized by backbone hydrogen bonds (purple), and continuous chains of side-chain hydrogen bonds (orange). The latter are crucial for packing the polar glutamines into the waterless amyloid core. When generating the core candidates, all the χ1 and χ3 dihedral angles were independently rotated to explore all potential hydrogen bond networks. B Stabilities (see Eq. (1) in “Methods”) of the 30 experimentally-feasible polyQ core candidates (represented by color-coded lines) as a function of MD simulation time. The three blue-shaded sections indicate the gentle protocol chosen for initiating simulations from the energy-minimized ideal structures: Decreasing position restraints (of 1 000, 500, and 100 kJ/mol/nm2) over three consecutive 100-ps periods led to the unrestrained simulation. Notably, only two candidates maintain stability throughout the 10-μs MD simulations; we denote these M1 and M2. C Atomic-level structures of the type “a” and “b” Glns in M2. Gln dihedral angle names shown on “b''. D The side-chain χ1–χ3 (left panels) and backbone ψ–ϕ (right) dihedral angle distributions of conformers “a” (red) and “b” (blue) for the final models M1 (top) and M2 (bottom). E Illustration of the inter-side-chain hydrogen-bond ladders (orange) in M2. F Nomenclature for Nϵ protons involved in the H-bonding along the ladder (HZ) or orthogonal to it (HE)46. G 2D 1H–15N MAS NMR spectrum on HTTex1 fibrils (see also Supplementary Fig. 7). Four cross-peaks are marked for the Gln side-chain NH2 protons of the “a” and “b” conformers that form the core. The dashed lines mark the 1H shifts for the HZ and HE protons, showing that they are identical for the two conformers. Panels (B–E) from the Amber14SB86 force field; for OPLSAA/M87 and CHARMM36m88 see Supplementary Figs. 4, 5. Source Data files for panels (B, D, and G) provided in ref. 85.