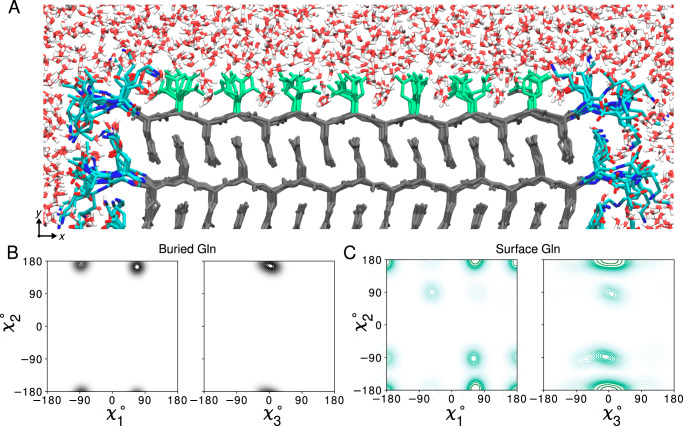
Fig. 4. Atomic model of the water-facing surface of polyQ amyloid.
A Atomistic MD snapshot of the D2Q15K2 peptide fibril’s polyQ surface in contact with water. Exposed and buried Gln residues are colored green and gray, respectively. Note how the Gln side-chains internal to the (model M1, for M2 see Supplementary Fig. 12) amyloid core are well-ordered, while the water-facing side-chains display more mobility. B Side-chain dihedral angle distributions for the buried Gln residues and (C) for the Gln residues on the fibril surface (Amber14SB86; for OPLSAA/M87 see Supplementary Figs. 13, 14). The surface-facing residues show more disorder, but are nonetheless constrained to just few varyingly prominent specific rotamer states. Source Data files for panels (B, C) provided in ref. 85.