Abstract
Adhesion of Plasmodium falciparum-infected erythrocytes to placental chondroitin 4-sulfate (CSA) has been linked to the severe disease outcome of pregnancy-associated malaria. Soluble polysaccharides that release mature-stage parasitized erythrocytes into the peripheral circulation may help elucidate these interactions and have the potential to aid in developing therapeutic strategies. We have screened a panel of 11 sulfated polysaccharides for their capacities to inhibit adhesion of infected erythrocytes to CSA expressed on CHO-K1 cells and ex vivo human placental tissue. Two carrageenans and a cellulose sulfate (CS10) were able to inhibit adhesion to CSA and to cause already bound infected erythrocytes to de-adhere in a dose-dependent manner. CS10, like CSA and in contrast to all other compounds tested, remained bound to infected erythrocytes after washing and continued to inhibit binding. Both carrageenans and CS10 inhibited adhesion to placental tissue. Although highly sulfated dextran sulfate can inhibit CSA-mediated adhesion to CHO cells, this polysaccharide amplified adhesion to placental tissue severalfold, demonstrating the importance of evaluating inhibitory compounds in systems as close to in vivo as possible. Interestingly, and in contrast to all other compounds tested, which had a random distribution of sulfate groups, CS10 exhibited a clustered sulfate pattern along the polymer chain, similar to that of the undersulfated placental CSA preferred by placental-tissue-binding infected erythrocytes. Therefore, the specific antiadhesive capacity observed here seems to depend not only on the degree of charge and sulfation but also on a particular pattern of sulfation.
Of the four Plasmodium species that are able to cause malaria in humans, infection with Plasmodium falciparum results in the most severe disease pathology and is the most lethal. The high pathogenicity of this parasite is partly due to the ability of mature trophozoite- and schizont-stage-infected erythrocytes to adhere to endothelial cells lining the capillaries and postcapillary venules, to adhere to the placental syncytiotrophoblast and in the intervillous space, to rosette with uninfected erythrocytes, or to autoagglutinate (7, 29, 35, 42). Such adhesive interactions are thought to contribute to the symptoms of severe malaria, cerebral malaria, and pregnancy-associated malaria (3, 28). Pregnancy-associated malaria in particular has been the focus of growing attention in recent years, especially with respect to vaccine and drug development (38). Pregnant women in areas of malaria endemicity undergoing their first pregnancy are more susceptible to this type of malaria, regardless of their previous level of exposure and immunity (8). However, multigravid women in these areas have been found to have a level of protection against pregnancy-associated malaria, and immune sera from these women are able to recognize placenta-binding infected erythrocytes from different geographical locations (19), making the parasite adhesin mediating placental binding a good vaccine and drug target.
The major P. falciparum adhesive proteins expressed on the surface of the infected erythrocyte are termed P. falciparum erythrocyte membrane protein 1 (PfEMP1). These antigenically variant surface proteins are products of a multigene family of which there are approximately 60 members per haploid genome (20). PfEMP1 is responsible for adhesion to a number of endothelial host cell proteins, including CD36, intercellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-1 (VCAM-1), E-selectin, P-selectin, and CD31 (reviewed in reference 17). Formation of rosettes is also PfEMP1 mediated, with complement receptor 1 (CR1) and CD36 identified as binding receptors (11, 15, 24, 36). In addition to protein receptors, sulfated polysaccharides have been shown to play a role in parasite rosetting (15) and sequestration. This is particularly evident for pregnancy-associated malaria, where the glycosaminoglycan chondroitin-4-sulfate (chondroitin sulfate A [CSA]) appears to be the major host receptor in the placenta (18). Another glycosaminoglycan, hyaluronic acid, has also been implicated as a placental receptor (6, 12).
Recent work has shown that chondroitin sulfate proteoglycans isolated from human placenta have unusually low levels of sulfation, with only ∼8% of the chondroitin sulfate (CS) chains being 4-sulfated and the remainder nonsulfated (1). Optimal binding of infected erythrocytes in the placenta requires ∼30% 4-sulfated and ∼70% nonsulfated disaccharide repeats, with a minimal binding motif of six disaccharide repeating units with two 4-sulfated and four nonsulfated disaccharide units (4). These findings were confirmed in a study using an immobilized commercially available bovine trachea C4S/C6S copolymer, although a binding motif was identified in which 4-sulfation was required in four or five of the six disaccharide repeating units instead of in two, as is the case for human placenta-derived C4S (13). The ability of infected erythrocytes to bind efficiently to low-sulfated chondroitin sulfate chains in the placental intervillous space remained puzzling until Achur and coworkers showed two different sulfation patterns where only 2 to 3% and 9 to 14% of the CS chain disaccharide units have 4-sulfation while the remainder are nonsulfated (2). The sulfate groups in the CS proteoglycans are clustered in CS chain domains of 6 to 14 repeating disaccharide units, with 20 to 28% 4-sulfation in the sulfate-rich regions of the CS chains and little or no sulfate in the remaining regions (2).
A variety of negatively charged polysaccharides have been tested for their capacity to inhibit invasion of human erythrocytes by P. falciparum merozoites and cytoadherence of parasitized erthryoctes to host receptors, including CD36 and CSA (16, 41), as well as to disrupt rosette formation of parasitized with uninfected erythrocytes (10, 34). From these studies it has become evident that complex sulfated polysaccharides such as heparins, dextran sulfates, fucoidan, and the nonsulfated glycosaminoglycan hyaluronic acid display inhibitory capacity to different extents. Here we have investigated the structural requirements for the antiadhesive capacity of polysaccharides by comparing the abilities of polysaccharides with differing levels and patterns of sulfation to disrupt infected-erythrocyte adhesion to CSA expressed on mammalian cells and the placenta. In addition to the known inhibitors such as CSA, fucoidan, and dextran sulfates, we have shown that two carrageenans and a cellulose sulfate with a unique sulfation pattern are able to inhibit CSA-specific adhesion of parasitized erythrocytes to CHO-K1 cells and human placenta.
MATERIALS AND METHODS
Reagents.
CSA from a bovine trachea was obtained from Sigma (St Louis, MO). Dextran sulfate from Leuconostoc species (500 kDa and 8 kDa), fucoidan from Fucus vesiculosus, and pectin from apple and citrus peels were all purchased from Sigma (St Louis, MO). Carrageenans CSW-2 (Lambda), LP-42 (Iota), and MB 73F (Kappa) were kindly provided by CP Kelco, Denmark. Pentosan polysulfate (xylan polysulfate) was purchased from Bene GmbH, Munich, Germany. Suramin (polysulfonated naphthylurea; 1,429.2 Da), a product of Bayer, Leverkusen, Germany, was kindly provided by G. Wirl (National Academy of Science, Salzburg, Austria). Cellulose sulfates (CS2, CS3, and CS10) were kindly provided by W. Wagenknecht, Fraunhofer Institute for Applied Polymer Research, Potsdam, Germany. Regioselective sulfation of these compounds was carried out as previously described. Briefly, CS3 was prepared directly from cellulose and CS2 from a nearly randomly acetylated cellulose-2.5-acetate (40). CS10 synthesis started with a cellulose triacetate (degree of polymerization of about 300, corresponding to a molecular mass of 50 kDa with respect to the cellulose backbone) which then was partially deacetylated with 1,6-diaminohexane and subsequently sulfated (30, 31). Structural analysis of the cellulose sulfates has been described elsewhere (21, 22). The chemical characteristics of the cellulose sulfates are listed in Table 1. The purity and absence of protein contamination in carbohydrate preparations were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis followed by Coomassie staining.
TABLE 1.
Structural parameters of polysaccharides used in this study
| Compounda | Gross structure | Mw (thousands)b | DSsulfatec | Position of sulfation in the sugar unit (S = SO3−) | Sulfation pattern along the polymer chaind |
|---|---|---|---|---|---|
| Inhibitory polysaccharides | |||||
| Chondroitin sulfate A (bovine trachea) | → 4)-β-d-GlcpA-(1 → 3)-β-d-GalpNAc-(1- | 50 | <0.50 | A: −, B: 4-S | Regular with unsulfated sequences |
| [A → B] | |||||
| Fucoidan (Fucus vesiculosus) | Mainly: → 2)-α-l-Fucp-(1- | 180 | ∼0.90 | Mainly 4-S-Fuc | assumed to be nearly regular |
| In addition: d-Gal, d-Xyl, d-HexA | |||||
| Dextran sulfate (500 kDa) (Leuconostoc spp.) | → 6)-α-d-Glcp-(1- | 500 | NKe | NK | Assumed to be random |
| Dextran sulfate (8 kDa) (Leuconostoc spp.) | → 6)-α-d-Glcp-(1- | 8 | NK | NK | Assumed to be random |
| λ-Carrageenan CSW-2 | → 3)-β-d-Galp-(1 → 4)-α-d-Galp-(1- | 230-790 | 1.05 | A: 70% 2-S, B: 2,6-di-S | Regular with respect to B |
| [A→B] | |||||
| ι-Carrageenan LP-42 | → 3)-β-d-Galp-(1 → 4)-3,6-anh-α-d-Galp-(1- | 230-790 | 0.75 | A: 4-S, B: 2-S | Regular |
| [A→B] | |||||
| Cellulose sulfate CS10 | → 4)-β-d-Glcp-(1- | ca. 50 | 1.30 | 44% in 2, 36% in 3, 20% in 6 | Regions of high sulfate density (clustered) |
| Noninhibitory polysaccharides | |||||
| κ-Carrageenan MB73F | → 3)-β-d-Galp-(1 → 4)-3,6-anh-α-d-Galp-(1- | 230-790 | 0.52 | A: 4-S, B: − | Regular |
| [A→B] | |||||
| Cellulose sulfate CS2 | → 4)-β-d-Glcp-(1- | ca. 30 | 0.35 | 22% in 2, 19% in 3, 59% in 6 | Random |
| Cellulose sulfate CS3 | → 4)-β-d-Glcp-(1- | NK | 0.52 | 9% in 2, 5% in 3, 86% in 6 | Random |
| Pectin | → 4)-α-d-GalpA-(1-, partially methyl esterified | 30-300 | NK | Assumed to be random | |
| → 2)-d-Rha-(1-, Ac | |||||
| Possible side chains: d-Xyl, → 5)-α-l-Araf-(1-, → 4)-β-d-Gal-(1- | |||||
| Xylan polysulfate | → 4)-[2-O-(4-O-Me-α-d-GlcpA)-Xylp]- (1 → 4)-β-d-Xylp-(1- | 5.7 | NK | Assumed to be random | |
| Suramin (polysulfonated naphthylurea)f | 8,8′-{Carbonylbis[imino-3,1-phenylene- carbononylimino(4-methyl-3,1-phenylene) carbonylimino]}bis-1,2,5-naphthalene- trisulfonic acid hexasodium | 1.429 |
Inhibitory polysaccharides inhibited adhesion of FCR3csa-infected erythrocytes to CHO-Kl cells at 100 μg/ml, while noninhibitory polysaccharides did not.
As given by the supplier (dextran, xylan, chondroitin, fucoidan), calculated from the degree of polymerization of the polysaccharide before sulfation (celluloses), or reported in the literature (carrageenans).
Average number of sulfated OH groups/sugar unit.
All products obtained by direct chemical sulfation can be assumed to exhibit a random sulfation pattern.
NK, not known.
Nonpolysaccharide.
P. falciparum and mammalian cell culture.
P. falciparum-infected erythrocytes were cultured in blood group A+ human erythrocytes and serum, as described by Trager and Jensen in 1976 (39), and maintained in a synchronous state by gelatin treatment (25). For studies of adhesion to CSA, the parasite clone FCR3 (37) was selected on CHO-K1 cells expressing chondroitin-4-sulfate and designated FCR3csa, as described previously (32). CHO-K1 cells (DSMZ, GmbH, Braunschweig, Germany) were cultured in RPMI 1640 medium containing 10% fetal calf serum and 1% penicillin-streptomycin (Gibco BRL). All parasite lines and cell cultures were free of mycoplasmas.
P. falciparum adhesion assays.
Cytoadhesion assays were carried out essentially as described previously (32). CHO-K1 cells were grown in 70 to 90% confluent monolayers in 24-well tissue culture plates. Trophozoite-infected erythrocytes were purified using gelatin flotation (25) and resuspended in RPMI 1640 pH 6.8 plus 10% human serum (5 × 106 parasitized erythrocytes/ml). For inhibition assays, infected erythrocytes were preincubated with test substances for 15 min at room temperature before being added to cell monolayers. Infected erythrocytes were allowed to adhere for 1 h at room temperature, with gentle agitation every 15 min, followed by washing to remove unbound infected erythrocytes. For de-adhesion assays, infected erythrocytes were first allowed to adhere to cell monolayers for 30 min. Then unbound infected erythrocytes were washed away; the bound infected erythrocytes were incubated with test substances for 1 h, with gentle agitation every 15 min; and the de-adhered infected erythrocytes were washed away. To determine whether substances bound irreversibly to infected erythrocytes, infected erythrocytes were incubated with different concentrations of the substance for 30 min and washed twice in RPMI 1640 medium, followed by adhesion to cell monolayers, as described above. All assays were carried out using RPMI 1640, pH 6.8, containing 10% human serum. As a control in each assay, specific adhesion to CSA was determined by adding 100 μg/ml of soluble CSA (Sigma). The number of infected-erythrocytes bound to 100 cells was calculated for at least 500 cells, and the percentage of binding was determined in comparison to the binding of controls. In each case three independent experiments were carried out.
Placental cryosection assays.
Binding assays on ex vivo placental tissue were carried out on unfixed sets of three consecutive 5-μm cryosections of normal human placenta mounted on microscope slides, as previously described (32). Placental tissue was obtained from delivering women after informed written consent, and with approval from the University of Heidelberg ethics committee on the use of human tissue in research according to the Helsinki protocol. Briefly, 70-μl volumes of trophozoite-infected erythrocytes resuspended at 5 × 106 parasites/ml in RPMI 1640 plus 10% human serum, pH 6.8, were preincubated for 15 min at room temperature with test compounds before being added to cryosections. Each consecutive set of three cryosections consisted of an untreated control, a CSA control (100 μg/ml), and the test compound (100 μg/ml). The average number of adherent infected erythrocytes (± standard error) per 40× high-power field was determined for cryosection sets from two different placental blocks, in two independent assays.
RESULTS
Adhesion inhibition and de-adhesion of FCR3csa-infected erythrocytes to CHO-K1 cells.
P. falciparum clone FCR3-infected erythrocytes were selected for a homogeneous CSA binding phenotype by repeated panning on CHO-K1 cells, as previously described (5). The abilities of different polysaccharides to inhibit the in vitro adhesion of FCR3csa-infected erythrocytes to CSA expressed on CHO-K1 cells were determined in a primary screen using 100 μg/ml of substances diluted in phosphate-buffered saline (PBS). Of the substances tested in the primary screen, a cellulose sulfate preparation, two carrageenans, fucoidan, and two dextran sulfates were able to completely inhibit the adhesion of FCR3csa-infected erythrocytes to CHO-K1 cells (Table 1). Nonsulfated, negatively charged polysaccharides (e.g., pectin), but also sulfated compounds such as xylan (pentosan) polysulfate and suramin, did not inhibit the adhesion of parasitized erythrocytes to CHO-K1 cells (Table 1). Similar results have been reported for suramin (4, 14), although there have been conflicting reports on the inhibitory capacity of xylan (pentosan) polysulfate (4, 14). In each assay, high levels of binding were observed for controls and binding was completely inhibited when soluble CSA was included as an inhibitor (not shown). For each inhibitory substance, the pH of the binding assay medium was the same as that in the control, excluding the possibility of pH-dependent inhibition.
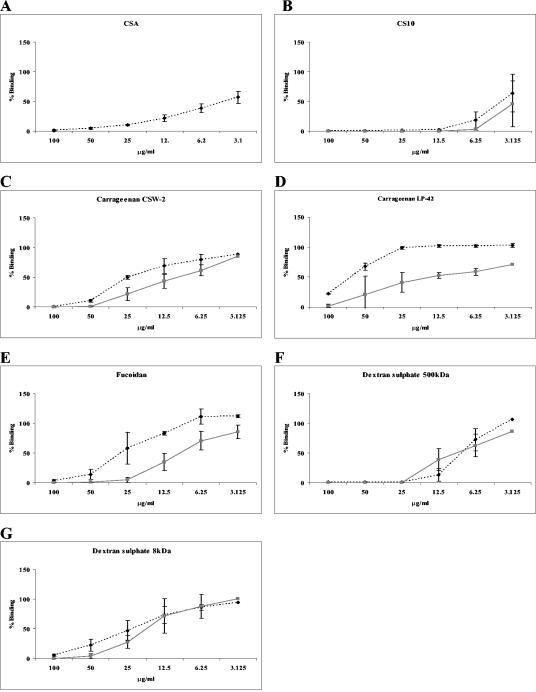
We next compared the CSA-specific adhesion inhibition and de-adhesion profiles of compounds found to affect adhesion in the primary screen. CSA, which inhibited adhesion at all concentrations tested (not shown), was also the most effective compound in de-adhesion assays, with ∼50% of bound infected erythrocytes de-adhering at a concentration of 3 μg/ml (Fig. 1A). Cellulose sulfate CS10 (Fig. 1B) was able to completely inhibit FCR3csa adhesion to CHO-K1 cells and to cause bound parasitized erythrocytes to de-adhere at concentrations greater than 12.5 μg/ml. Although less effective than cellulose sulfate, three of four seaweed extracts tested (carrageenan types CSW-2 and LP-42, and fucoidan) also inhibited adhesion and caused bound parasitized erythrocytes to de-adhere in a dose dependent manner (Fig. 1C, D, and E). Similar adhesion inhibition profiles were obtained for carrageenans CSW-2 and LP-42; however, LP-42 was less efficient at causing bound infected erythrocytes to de-adhere (Fig. 1C and D). Fucoidan was also less effective at causing bound infected erythrocytes to de-adhere than at directly inhibiting adhesion (Fig. 1E). The abilities of 500-kDa and 8-kDa dextran sulfate (Fig. 1F and G) to inhibit adhesion and to cause bound infected erythrocytes to de-adhere were similar, although the 500-kDa form of dextran sulfate showed a greater effect than the 8-kDa form.
FIG. 1.
Adhesion of FCR3csa-infected erythrocytes to CHO-K1 cells. Varying concentrations of test substances were used either to inhibit binding (░⃞) or to cause already bound infected erythrocytes to de-adhere (⧫). Mean percentages of binding (± standard deviations) compared to that of matched PBS controls (100% binding) for three independent experiments are shown.
Placental adhesion inhibition.
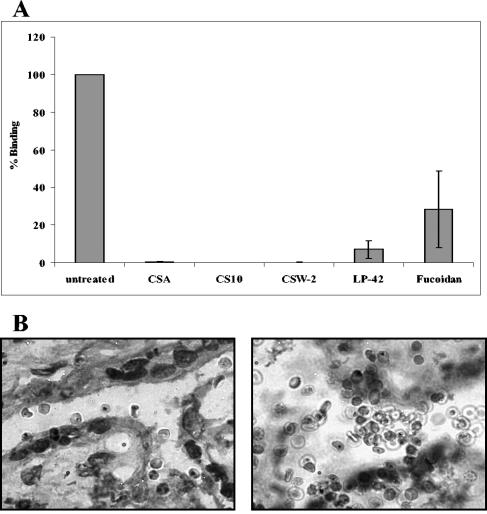
All six inhibitory substances were tested for inhibition of adhesion of FCR3csa-infected erythrocytes to unfixed human placental cryosections. Cellulose sulfate CS10 and both carrageenans (CSW-2 and LP-42) were able to completely abolish adhesion to the placental syncytiotrophoblast (Fig. 2A). Inhibition of adhesion by fucoidan was more variable, with a ∼72% (±51%) reduction in binding compared to that of untreated sections (Fig. 2A). Similar results were obtained when the number of infected erythrocytes adhering to the intervillous space on the cryosections was compared to that of PBS controls (not shown). CSA was included as a control in each assay and was able to completely inhibit binding to both the syncytiotrophoblast (Fig. 2A) and the intervillous space (not shown). In contrast, 500-kDa and 8-kDa dextran sulfate showed an unusual result when tested for adhesion inhibition on placental cryosections. While these carbohydrates were able to completely inhibit adhesion of FCR3csa-infected erythrocytes to CHO-K1 cells, a large accumulation of infected and noninfected erythrocytes was observed on placental cryosections, primarily in the intervillous space (Fig. 2B, right). This accumulation of cells did not occur on areas of the glass microscope slide that contained no placental tissue. Apparently the dextran sulfate was able to form a “sticky matrix,” trapping both infected and uninfected erythrocytes alike.
FIG. 2.
Inhibition of adhesion of FCR3csa-infected erythrocytes to ex vivo placental cryosections. (A) Effect of 100 μg/ml of each substance on adhesion of infected erythrocytes to sets of three consecutive placental cryosections. Mean percentages of binding to the syncytiotrophoblast compared to that of controls (± standard deviations) for four experiments are shown. (B) Giemsa-stained placental cryosections (magnification, ×1,000) showing adherent FCR3csa-infected erythrocytes (left) and accumulation of FCR3csa-infected and uninfected erythrocytes in the presence of 100 μg/ml 500-kDa dextran sulfate (right).
Binding of polysaccharides to infected erythrocytes.
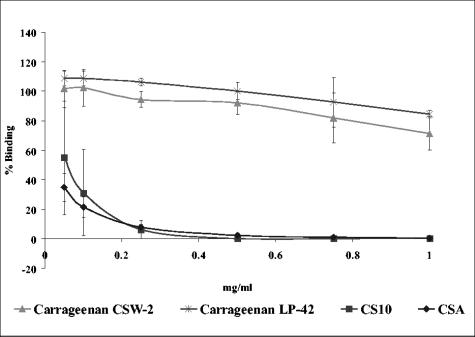
In order to test whether substances able to interfere with CSA adhesion bind to the parasitized erythrocyte, we preincubated FCR3csa-infected erythrocytes with test substances and then washed them extensively before carrying out adhesion assays on CHO-K1 cells. Cellulose sulfate CS10 yielded the best result, giving an adhesion profile similar to that of CSA, although there was some variation between experiments at the lowest concentrations tested (Fig. 3). In contrast, no significant inhibition of binding was observed for the two carrageenans, CSW-2 and LP-42, indicating that these substances were not remaining bound to the infected erythrocytes (Fig. 3). Similar results were obtained for fucoidan and the two dextran sulfates (not shown).
FIG. 3.
Adhesion of FCR3csa-infected erythrocytes to CHO-K1 cells after preincubation with polysaccharides and extensive washing. FCR3csa-infected erythrocytes were preincubated with varying concentrations of test compounds, washed, and allowed to adhere to CHO-K1 cells. Mean percentages of binding (± standard deviations) compared to that of matched PBS controls (100% binding) for three independent experiments are shown.
DISCUSSION
The ability of P. falciparum-infected erythrocytes to adhere to receptors on the hosts' microvascular endothelium and in the placenta has been linked with severe malarial pathology and associated disease outcomes, such as those observed in individuals with cerebral or placental malaria. The application of soluble polysaccharides as inhibitors of carbohydrate-mediated interactions, such as binding of infected erythrocytes to placental CSA, may help in elucidating the molecular requirements for these adhesive processes and could potentially lead to an intervention strategy. In order to investigate this, we have screened a panel of 11 polysaccharides with varying sulfation levels and charges for their abilities to interfere with the binding of P. falciparum-infected erythrocytes to CSA expressed on an in vitro cell model and to human placenta.
Of all the polysaccharides examined, we achieved the most promising results for one of three cellulose sulfate derivatives tested. CS10, but not CS2 or CS3, was able to inhibit adhesion of infected erythrocytes to CSA expressed on CHO-K1 cells and to cause bound infected erythrocytes to de-adhere from the surfaces of CHO-K1 cells. The de-adhesion profile obtained for CS10 was similar to that obtained for CSA, although CSA was better at directly inhibiting adhesion to CHO-K1 cells. CS10 was found to remain bound to the infected erythrocyte even after extensive washing and to abrogate adhesion to placental cryosections. The structural characteristics of CS10 compared to those of CS2 and CS3 appear to account for the specific inhibitory capacity observed. Perhaps the most important difference is related to the sulfation pattern along the polymer chain. While in CS2 and CS3 sulfate groups are distributed in a random manner, CS10 has a tendency to a more clustered/block-like pattern due to a different strategy of synthesis using cellulose triacetate as the starting material, as outlined in Materials and Methods. The location of the sulfate groups depends on the outcome of the deacetylation procedure, which is regioselective in the case of CS10, while direct sulfation always generates a random pattern due to reversibility and thermodynamic control. The clustered distribution of sulfate groups in CS10 resembles that of placental chondroitin, with low sulfation levels where four to five disaccharide units with 4-O-sulfate groups alternate with one to two nonsulfated disaccharides. Therefore, it seems that a distinct pattern of sulfation rather than a high overall and even distribution of sulfate groups governs specific interactions with P. falciparum-infected erythrocytes. Further, CS10 has a much higher charge/mass ratio, with a degree of substitution (DS) of 1.30 in comparison to CS2 and CS3, which show comparably low overall degrees of sulfation (DS, 0.35 and 0.52, respectively [Table 1]). A further structural difference is the regioselectivity of sulfation: CS2 and CS3 are preferably sulfated at the primary O-6 position, and CS10 exhibits high sulfation at the secondary O-2 and O-3 groups. It has been shown that selective removal of O-6-sulfation in CSA enhances chondroitin sulfate-mediated adhesion (13). Therefore, a lower degree of O-6-sulfation may also contribute to the results observed in this study. This pattern is also known to be important for the blood-coagulating activity of cellulose sulfates (23).
Of the three carageenans tested in this study, two were found to inhibit adhesion to CSA expressed on CHO cells and to cause CSA-binding infected erythrocytes to de-adhere in a dose-dependent manner. Adhesion inhibition appeared to be dependent on the type and level of sulfation, as MB73F (κ-carrageenan), which has a low level of sulfation compared to the other two carrageenans (Table 1), failed to inhibit (approximate charge/dimer: κ-carrageenan, 1.03; ι-carrageenan, 1.49; λ-carrageenan, 2.09 [9]). Differences in monosaccharide composition, in particular in the content of 3,6-anhydrogalactose-2-sulfate, may also account for differences in biological activity (κ-carrageenan has 0.82 molecule of anhydrogalactose/dimer, ι-carrageenan has 0.59, and λ-carrageenan has 0.16). Carrageenans LP-42 (ι-carrageenan) and CSW2 (λ-carrageenan) were also effective at blocking CSA-specific adhesion to human placenta, although the activities of these polysaccharides are apparently nonspecific or easily reversible (Fig. 3).
Dextran sulfates and fucoidan have previously been shown to inhibit the adhesion of infected erythrocytes to CD36 (41) and to disrupt rosettes (10, 34). In this study we have shown that these polysaccharides also inhibit CSA-specific adhesion to CHO cells and cause CSA-binding infected erythrocytes to de-adhere (Fig. 2). This finding is in contrast to that of Rogerson et al. (33), who observed no inhibition by these polysaccharides using infected erythrocytes of the same lineage as that used in this study (33). At the concentration of dextran sulfate and fucoidan used by Rogerson et al. (10 μg/ml), we observed ∼50% binding compared to that of untreated controls. The apparent differences between the two studies may, however, be due to the molecular mass of dextran sulfate used. We found that the inhibition and de-adhesion activities of the 8-kDa dextran sulfate were lower than those of the 500-kDa dextran sulfate, suggesting that both size and charge may play a role in interfering with infected-erythrocyte adhesion to CSA. When the effects of the two dextran sulfates on CSA-specific adhesion to placental cryosections were examined, the adhesion of infected erythrocytes and uninfected erythrocytes, which do not normally adhere to the placental sections, was amplified. These highly sulfated polysaccharides are apparently forming a sticky matrix on the ex vivo placental tissue to which both uninfected and infected erythrocytes can adhere. A similar finding has been reported previously for CD36-specific adhesion to in vitro-cultured cells. Heparin, fucoidan, and 500-kDa and 5-kDa dextran sulfates all promoted the adhesion of infected erythrocytes to dermal microvascular endothelial cells and CD36-transfected COS cells but not to purified platelet CD36 immobilized on plastic (27).
Our findings that CS10 specifically interferes with adhesion to placental CSA and that in vitro inhibition results are comparable with those obtained with soluble CSA provide further insight into the molecular requirements for adhesion to this major placental receptor. The specific antiadhesive capacity observed here seems to depend not only on the degree of charge and sulfation but also on a particular pattern of sulfation. Unlike carrageenans and dextran sulfates, which are known to be inducers of inflammatory processes (26), to our knowledge no negative effects of cellulose sulfate on vital cellular systems have been reported, making CS10 a potential candidate in the search for a treatment of pregnancy-associated malaria.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) SFB 544 “Control of Tropical Infectious Diseases.”
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Achur, R. N., M. Valiyaveettil, and D. C. Gowda. 2003. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. J. Biol. Chem. 278:11705-11713. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa, M., M. Iseki, J. W. Barnwell, D. Taylor, M. M. Oo, and R. J. Howard. 1990. The pathology of human cerebral malaria. Am. J. Trop. Med. Hyg. 43:30-37. [DOI] [PubMed] [Google Scholar]
- 4.Alkhalil, A., R. N. Achur, M. Valiyaveettil, C. F. Ockenhouse, and D. C. Gowda. 2000. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 275:40357-40364. [DOI] [PubMed] [Google Scholar]
- 5.Andrews, K. T., N. K. Viebig, F. Wissing, N. Klatt, N. Oster, H. Wickert, P. Knolle, and M. Lanzer. 2003. A human schwannoma cell line supports the in vitro adhesion of Plasmodium falciparum infected erythrocytes to chondroitin-4-sulfate. Parasitol. Res. 89:188-193. [DOI] [PubMed] [Google Scholar]
- 6.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder, W. Chai, A. M. Lawson, M. E. Molyneux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berendt, A. R., D. J. Ferguson, J. Gardner, G. Turner, A. Rowe, C. McCormick, D. Roberts, A. Craig, R. Pinches, B. C. Elford, et al. 1994. Molecular mechanisms of sequestration in malaria. Parasitology 108(Suppl.):S19-S28. [DOI] [PubMed] [Google Scholar]
- 8.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 9.Caram-Lelham, N., and L.-O. Sundelof. 1995. Some aspects on characterization and properties of charged polysaccharides. An investigation of the system carrageenan/amitriptyline/water with the relation to amiphiphile adsorption and charge density. Int. J. Pharm. 115:103-111. [Google Scholar]
- 10.Carlson, J., H. P. Ekre, H. Helmby, J. Gysin, B. M. Greenwood, and M. Wahlgren. 1992. Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparin devoid of anticoagulant activity. Am. J. Trop. Med. Hyg. 46:595-602. [DOI] [PubMed] [Google Scholar]
- 11.Carlson, J., and M. Wahlgren. 1992. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J. Exp. Med. 176:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai, W., J. G. Beeson, H. Kogelberg, G. V. Brown, and A. M. Lawson. 2001. Inhibition of adhesion of Plasmodium falciparum-infected erythrocytes by structurally defined hyaluronic acid dodecasaccharides. Infect. Immun. 69:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai, W., J. G. Beeson, and A. M. Lawson. 2002. The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. J. Biol. Chem. 277:22438-22446. [DOI] [PubMed] [Google Scholar]
- 14.Chaisavaneeyakorn, S., P. Kongtawelert, P. Angkasekwinai, R. McGready, F. Nosten, and S. C. Chaiyaroj. 2004. Inhibitory activities of sulfated proteoglycans on chondroitin sulfate A-mediated cytoadherence of Plasmodium falciparum isolates from Thailand. Am. J. Trop. Med. Hyg. 70:149-157. [PubMed] [Google Scholar]
- 15.Chen, Q., A. Barragan, V. Fernandez, A. Sundstrom, M. Schlichtherle, A. Sahlen, J. Carlson, S. Datta, and M. Wahlgren. 1998. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J. Exp. Med. 187:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark, D. L., S. Su, and E. A. Davidson. 1997. Saccharide anions as inhibitors of the malaria parasite. Glycoconj. J. 14:473-479. [DOI] [PubMed] [Google Scholar]
- 17.Craig, A., and A. Scherf. 2001. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol. Biochem. Parasitol. 115:129-143. [DOI] [PubMed] [Google Scholar]
- 18.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 19.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 20.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gohdes, M., and P. Mischnick. 1998. Determination of the substitution pattern in the polymer chain of cellulose sulfates. Carbohydr. Res. 309:109-115. [Google Scholar]
- 22.Gohdes, M., P. Mischnick, and W. Wagenknecht. 1997. Methylation analysis of cellulose sulphates. Carbohydr. Polym. 33:163-168. [Google Scholar]
- 23.Groth, T., and W. Wagenknecht. 2001. Anticoagulant potential of regioselective derivatized cellulose. Biomaterials 22:2719-2729. [DOI] [PubMed] [Google Scholar]
- 24.Handunnetti, S. M., M. R. van Schravendijk, T. Hasler, J. W. Barnwell, D. E. Greenwalt, and R. J. Howard. 1992. Involvement of CD36 on erythrocytes as a rosetting receptor for Plasmodium falciparum-infected erythrocytes. Blood 80:2097-2104. [PubMed] [Google Scholar]
- 25.Jensen, J. B. 1978. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 27:1274-1276. [DOI] [PubMed] [Google Scholar]
- 26.Kim, H., and A. Berstad. 1992. Experimental colitis in animal models. Scand. J. Gastroenterol. 27:529-537. [DOI] [PubMed] [Google Scholar]
- 27.McCormick, C. J., C. I. Newbold, and A. R. Berendt. 2000. Sulfated glycoconjugates enhance CD36-dependent adhesion of Plasmodium falciparum-infected erythrocytes to human microvascular endothelial cells. Blood 96:327-333. [PubMed] [Google Scholar]
- 28.Menendez, C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today 11:178-183. [DOI] [PubMed] [Google Scholar]
- 29.Miller, L. H., and J. D. Smith. 1998. Motherhood and malaria. Nat. Med. 4:1244-1245. [DOI] [PubMed] [Google Scholar]
- 30.Philipp, B., D. Klemm, and A. Stein. 1995. Regioselektive Veresterung und Veretherung von Cellulose und Cellulosederivaten. Teil 3. Synthese regioselekiv substituierter Celluloseether und zusammenfassenede Diskussion. Papier 49:102-108. [Google Scholar]
- 31.Philipp, B., D. Klemm, and W. Wagenknecht. 1995. Regioselektive Veresterung und Veretherung von Cellulose und Cellulosederivaten. Teil 2. Synthese regioselektiv substituierter Celluloseester. Papier 49:58-64. [Google Scholar]
- 32.Pouvelle, B., P. Meyer, C. Robert, L. Bardel, and J. Gysin. 1997. Chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of Plasmodium falciparum infected erythrocytes. Mol. Med. 3:508-518. [PMC free article] [PubMed] [Google Scholar]
- 33.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe, A., A. R. Berendt, K. Marsh, and C. I. Newbold. 1994. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Exp. Parasitol. 79:506-516. [DOI] [PubMed] [Google Scholar]
- 35.Rowe, A., J. Obeiro, C. I. Newbold, and K. Marsh. 1995. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 63:2323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe, J. A., J. M. Moulds, C. I. Newbold, and L. H. Miller. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388:292-295. [DOI] [PubMed] [Google Scholar]
- 37.Scherf, A., R. Hernandez-Rivas, P. Buffet, E. Bottius, C. Benatar, B. Pouvelle, J. Gysin, and M. Lanzer. 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 17:5418-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, J. D., and K. W. Deitsch. 2004. Pregnancy-associated malaria and the prospects for syndrome-specific antimalaria vaccines. J. Exp. Med. 200:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 40.Wagenknecht, W., I. Nehls, and B. Philipp. 1993. Studies on the regioselectivity of cellulose sulfation in an N2O4-N,N-dimethylformamide-cellulose system. Carbohydr. Res. 240:245-252. [Google Scholar]
- 41.Xiao, L., C. Yang, P. S. Patterson, V. Udhayakumar, and A. A. Lal. 1996. Sulfated polyanions inhibit invasion of erythrocytes by plasmodial merozoites and cytoadherence of endothelial cells to parasitized erythrocytes. Infect. Immun. 64:1373-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada, M., R. Steketee, C. Abramowsky, M. Kida, J. Wirima, D. Heymann, J. Rabbege, J. Breman, and M. Aikawa. 1989. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am. J. Trop. Med. Hyg. 41:161-168. [DOI] [PubMed] [Google Scholar]