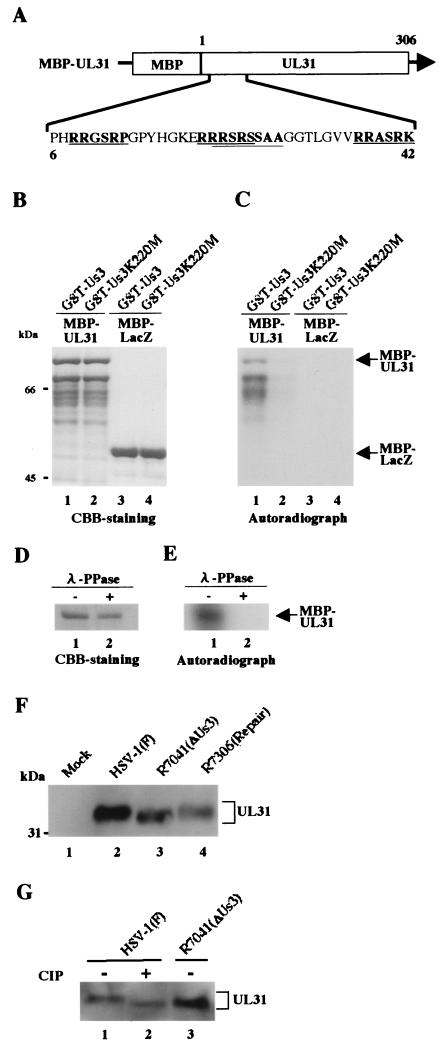
FIG. 4.
(A) Schematic diagram of the amino acid sequence of UL31 fused to MBP. The amino acid sequence of UL31 residues 6 to 42 is shown. Sites with the consensus sequence for phosphorylation by Us3 are underlined. (B) CBB-stained and autoradiographic images of purified MBP-UL31 (lanes 1 and 2) and MBP-lacZ (lanes 3 and 4) incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 (lanes 1 and 3) or GST-Us3K220M (lanes 2 and 4), separated on a denaturing gel, and stained with CBB. Molecular masses (kDa) are shown on the left. (C) Autoradiograph of the gel in panel B. (D) Purified MBP-UL31 incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 was then either mock treated (lane 1) or treated with λ-PPase (lane 2), separated on a denaturing gel, and stained with CBB. (E) Autoradiograph of the gel in panel D. (F) An immunoblot of electrophoretically separated lysates from Vero cells mock infected (lane 1) or infected with HSV-1(F) (lane 2), R7041 (lane 3), or R7306 (lane 4) at multiplicities of infection of 3 PFU per cell. The infected cells were harvested at 12 h after infection and subjected to immunoblotting with a polyclonal antibody to UL31 (37). Molecular masses (kDa) are shown on the left. (G) The lysates used for panel F were mock treated (lanes 1 and 3) or treated with alkaline phosphatase (lane 2), separated on a denaturing gel, and subjected to immunoblotting with the antibody to UL31.