Table 1.
Affinities or Antagonistic Activities of Triazoloquinazoline Derivatives in Radioligand Binding Assays at A1, A2A, and A3 Receptorsa–c
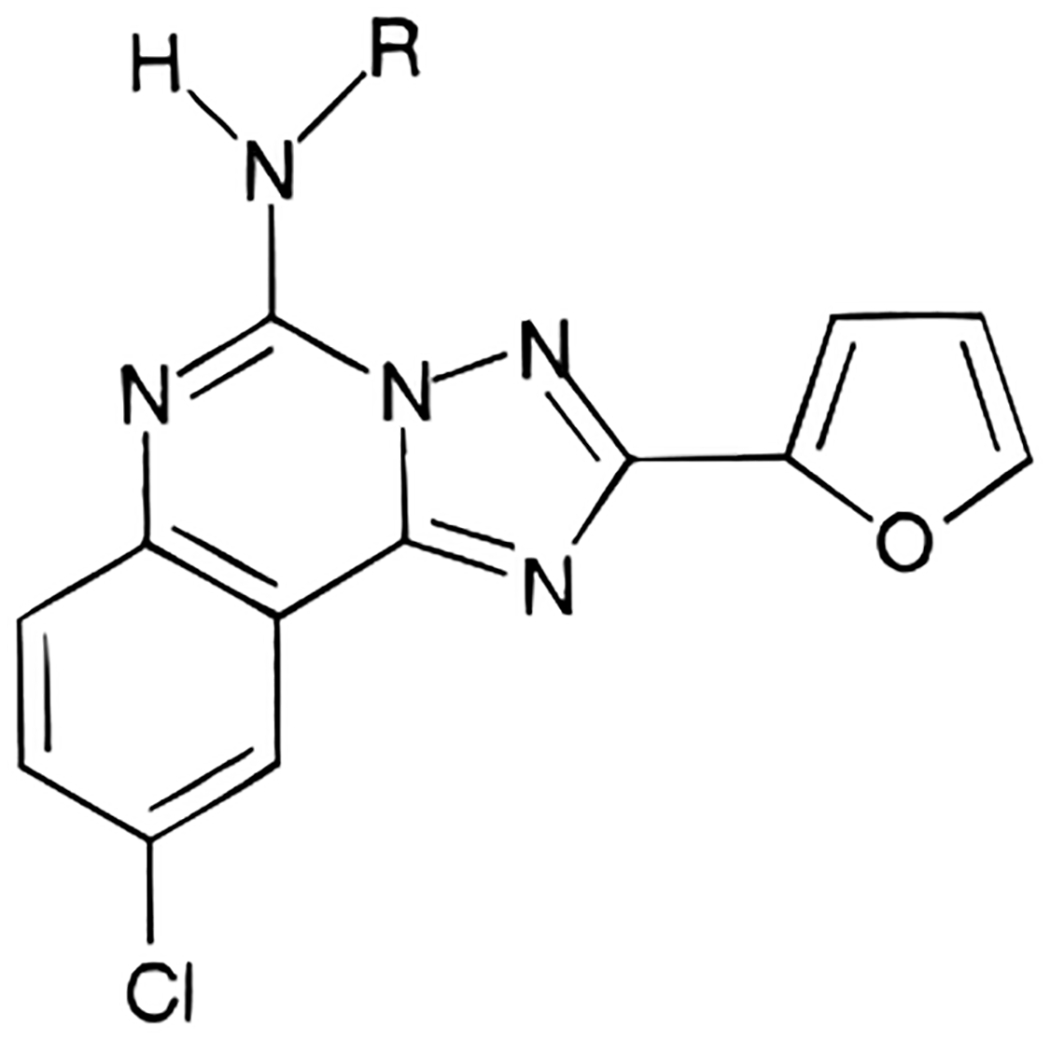
| ||||||
|---|---|---|---|---|---|---|
| compd | R | Ki or IC50 (nM) | ||||
| rA1 | rA2A | hA3 | rA1/hA3 | rA2A/hA3 | ||
| 1a (CGS 15943) | H | 21 ± 3.0d | 3.3 ± 1.7d | 13.8 ± 2.4 | ||
| 1b | COCH2-Ph | 52.7 ± 11.8e | 10.3 ± 3.7e | 0.65 ± 0.25 | 81 | 16 |
| 3 | CH2-Ph | 1200 ± 15 | 200 ± 34 | 42.5 ± 6.91 | 28 | 4.7 |
| 4 | COCH2-(4-CH3O-Ph) | 30.2 ± 6.3 | 28.0 ± 4.8 | 14.4 ± 3.2 | 2.1 | 1.9 |
| 5 | COCH2-(4-NH2-3-I-Ph) | 217 ± 65 | 10.4 ± 2.2 | 49.3 ± 17.9 | 4.4 | 0.21 |
| 6 | COCH2-(4-NH2-Ph) | 24.9 ± 7.7 | 6.97 ± 1.13 | 3.56 ± 1.24 | 7.0 | 2.0 |
| 7 | COCH2-(2-I-Ph) | 2300 ± 590 | 17.2 ± 4.0 | >10000 | <1 | ≪1 |
| 8 | COCH2-(3-I-Ph) | 45.4 ± 8.7 | 9.67 ± 1.74 | 882 ± 242 | 0.051 | 0.011 |
| 9 | COCH2-(4-I-Ph) | 13.8 ± 4.6 | 9.93 ± 2.00 | 62.9 ± 13.0 | 0.22 | 0.16 |
| 10 | COCH2-(3-Cl-Ph) | 43.0 ± 6.4 | 9.43 ± 2.65 | 32.1 ± 11.3 | 1.3 | 0.29 |
| 11 | (R)-COCH(CH3)(Ph) | 46.2 ± 11.9 | 11.1 ± 1.3 | 0.362 ± 0.053 | 130 | 31 |
| 12 | (S)-COCH(CH3)(Ph) | 37.5 ± 8.5 | 20.1 ± 3.7 | 0.468 ± 0.111 | 80 | 43 |
| 13 | COCH(Ph)2 | 129 ± 43 | 12.1 ± 3.4 | 0.586 ± 0.196 | 220 | 21 |
| 14 | COC(CH3)(Ph)2 | 890 ± 131 | 453 ± 156 | 194 ± 42 | 4.6 | 2.3 |
| 15 | COCH2CH2-Ph | 45.2 ± 7.5 | 28.3 ± 10.3 | 23.6 ± 7.6 | 1.9 | 1.6 |
| 16 | COCH=CH-Ph (trans) | 282 ± 71 | 59.8 ± 13.4 | 72.1 ± 15.6 | 3.9 | 0.71 |
| 17 | d-COCH(CH3)(NH-Boc) | 48.6 ± 6.7 | 18.8 ± 5.3 | 46.3 ± 5.4 | 1.0 | 0.41 |
| 18 | L-COCH(CH3)(NH-Boc) | 54.7 ± 13.4 | 6.65 ± 1.56 | 82.9 ± 2.7 | 0.66 | 0.080 |
| 19 | CO(CH2)2-NH-Boc | 31.0 ± 2.2 | 7.58 ± 0.80 | 6.71 ± 0.67 | 4.6 | 1.1 |
| 20 | CO(CH2)3-NH-Boc | 45.9 ± 13.2 | 19.9 ± 4.6 | 32.9 | 1.4 | 0.60 |
| 21 | CO(CH2)4-NH-Boc | 30.1 ± 6.8 | 3.64 ± 0.34 | 22.0 ± 3.1 | 1.4 | 0.17 |
| 22 | CO(CH2)5-NH-Boc | 53.8 ± 12.7 | 33.7 ± 7.3 | 33.8 ± 10.2 | 1.6 | 1.0 |
| 23 | CO(CH2)6-NH-Boc | 38.2 ± 2.6 | 11.1 ± 1.9 | 53.7 ± 31.0 | 0.71 | 0.21 |
| 24 | d-COCH(CH3)(NH2) | 193 ± 17 | 29.7 ± 9.1 | 1140 ± 370 | 0.17 | 0.026 |
| 25 | l-COCH(CH3)(NH2) | 390 ± 127 | 143 ± 13 | 1200 ± 460 | 0.33 | 0.12 |
| 26 | CO(CH2)2-NH2 | 89.8 ± 23.3 | 7.58 ± 2.1 | 874 ± 4 | 0.10 | 0.0088 |
| 27 | CO(CH2)3-NH2 | 8.75 ± 2.28 | 1.38 ± 0.23 | 80.8 ± 7.4 | 0.11 | 0.017 |
| 28 | CO(CH2)4-NH2 | 6.99 ± 1.38 | 1.13 ± 0.41 | 57.9 ± 20.8 | 0.12 | 0.020 |
| 29 | CO(CH2)5-NH2 | 99.6 ± 6.7 | 10.3 ± 3.7 | 213 ± 27 | 0.47 | 0.048 |
| 30 | CO(CH2)6-NH2 | 114 ± 26 | 55.9 ± 5.5 | 346 ± 77 | 0.33 | 0.16 |
| 31 | CO(CH2)4-COOBn | 304 ± 126 | 16.7 ± 2.5 | 44.7 ± 14.1 | 6.8 | 0.37 |
| 32 | CO(CH2)2-COOCH3 | 71.8 ± 7.2 | 28.8 ± 10.7 | 55.1 ± 8.6 | 1.3 | 0.52 |
| 33 | CO(CH2)6-COOCH3 | 46.6 ± 10.5 | 7.43 ± 2.72 | 59.0 ± 18.1 | 0.79 | 0.13 |
| 34 | CO(CH2)3-COOH | 45 ± 4 | 1.15 ± 0.47 | 81.3 ± 11.0 | 0.55 | 0.014 |
Displacement of specific [3H]R-PIA binding in rat brain membranes, expressed as Ki ± SEM (n = 3–5).
Displacement of specific [3H]CGS 21680 binding in rat striatal membranes, expressed as Ki ± SEM (n = 3–6).
Displacement of specific [125I]AB-MECA binding at human A3 receptors expressed in HEK cells, in membranes, expressed as Ki ± SEM (n = 3–4).
IC50 values for 1a (see ref 20).
Previously reported Ki values were 305 and 52.0 nM for rat A1 and A2A receptors, respectively.20
