Abstract
The bovine papillomavirus E2 protein maintains and segregates the viral extrachromosomal genomes by tethering them to cellular mitotic chromosomes. E2 interacts with a cellular bromodomain protein, Brd4, to mediate the segregation of viral genomes into daughter cells. Brd4 binds acetylated histones and has been observed to diffusely coat mitotic chromosomes in several cell types. In this study, we show that in mitotic C127 cells, Brd4 diffusely coated the condensed chromosomes. However, in the presence of the E2 protein, E2 and Brd4 colocalized in punctate dots that were randomly distributed over the chromosomes. A similar pattern of E2 and Brd4 colocalization on mitotic chromosomes was observed in CV-1 cells, whereas only a faint chromosomal coating of Brd4 was detected in the absence of the E2 protein. Therefore, the viral E2 protein relocalizes and/or stabilizes the association of Brd4 with chromosomes in mitotic cells. The colocalization of E2 and Brd4 was also observed in interphase cells, indicating that this protein-protein interaction persists throughout the cell cycle. The interaction of E2 with Brd4 greatly stabilized the association of Brd4 with interphase chromatin. In both mitotic and interphase cells, this stabilization required a transcriptionally competent transactivation domain, but not the DNA binding function of the E2 protein. Thus, the E2 protein modulates the chromatin association of Brd4 during both interphase and mitosis. This study demonstrates that the segregation of papillomavirus genomes is not simply due to the passive hitchhiking of the E2/genome complex with a convenient cellular chromosomal protein.
Persistent papillomavirus infections are established in the mitotically active basal cells of the squamous epithelium. Certain papillomaviruses, including bovine papillomavirus type 1 (BPV-1), can also infect fibroblast cells. Within these dividing cells, the viral genome is amplified to a low copy number and stably maintained as a persistent extrachromosomal element in the cell nucleus. As infected cells undergo division, papillomaviruses, like other extrachromosomal viruses, must position their genomes in such a way as to ensure an equal distribution of viral DNA to daughter cells and to avoid loss following nuclear membrane disassembly. BPV-1 has served as a model with which to study this process in papillomaviruses due to its ability to efficiently replicate and maintain the viral genome extrachromosomally through many cell divisions (26).
BPV-1 viral genomes are tethered to cellular mitotic chromosomes via a protein-protein interaction mediated by the viral protein E2 (20, 29, 50). By this action, viral genomes are efficiently segregated into daughter cells, thus ensuring the longevity of the viral infection. The central role of the viral E2 protein in mitotic tethering and genome segregation has been well documented (2, 20, 29, 45, 50). Pirsoo et al. first demonstrated that both the E2 protein and E2 DNA binding sites are required for long-term extrachromosomal genome maintenance (45). Subsequently, the BPV-1 genomes and the E2 protein were shown to be associated with mitotic chromosomes (2, 29, 50), followed by the observation that in the absence of this association, viral genomes were quickly lost (20, 29). The genomes of other extrachromosomal viruses, such as Epstein-Barr virus and human herpesvirus 8 (HHV-8), are also noncovalently attached to cellular chromosomes via interactions between viral and cellular proteins to facilitate genome segregation and maintenance (1, 15, 16).
The papillomavirus E2 protein also functions as a transcriptional regulator of viral gene expression and, in complex with the viral E1 protein, initiates viral DNA replication (reviewed in reference 36). The full-length E2 transactivator protein (E2-TA) consists of an N-terminal transactivation domain which is separated from the C-terminal DNA binding and dimerization domain by a flexible hinge region (reviewed in reference 36). The transactivation domain is essential for the segregation, transcriptional regulation, and replication functions of E2 and mediates the association of this viral protein with several cellular factors. These factors include components of the basal transcription and replication machinery (30, 47, 51, 55), transcriptional coactivators, and chromatin remodeling complexes (8, 27, 28, 40, 41). The E2 open reading frame of BPV-1 also encodes two smaller proteins, E2-TR and E8/E2, which lack the transactivation domain, are unable to bind mitotic chromosomes, and instead act as repressors of transcription (9, 25). These repressors antagonize the E2-TA function by competing for E2 DNA binding sites and sequestering E2-TA in inactive heterodimers (reviewed in reference 37).
A recent study by You et al. showed that E2 interacts with the cellular factor Brd4 and identified Brd4 as the tethering factor involved in the mitotic association of BPV-1 genomes (56). Brd4 is a member of the BET subgroup of the bromodomain protein family (reviewed in reference 12). Members of this subgroup have two bromodomains and an additional ET (extraterminal protein interaction) domain, both of which are conserved from Saccharomyces cerevisiae to humans (17, 21). Brd4 has been implicated in the regulation of cell growth and cell cycle progression (11, 18, 22, 33). A striking feature of Brd4 is its ability to bind acetylated lysines of histones H3 and H4 and remain associated with chromatin throughout mitosis, thus making it a strong candidate as the cellular partner of the E2 protein (10). Notably, Brd2, another member of the BET subgroup with homology to Brd4, has previously been identified as a chromosome-bound cellular factor capable of interacting with LANA, the viral protein responsible for linking HHV-8 genomes to mitotic chromosomes (34, 46).
Using a panel of E2 proteins with mutations in the transactivation domain, recent studies in our laboratories have established a strong correlation between the ability of the E2 protein to bind mitotic chromosomes and its capacity to interact in vitro with Brd4 (3). All proteins capable of associating with mitotic chromosomes could also bind Brd4, and Brd4 binding was abrogated for E2 proteins that could not associate with mitotic chromosomes (3). Furthermore, we have shown that E2-mediated plasmid segregation in yeast can be reconstituted by the expression of Brd4 (7).
For this study, we analyzed the association of E2 and Brd4 protein complexes with chromatin and the nuclear matrix at different stages of the cell cycle by comparing their extraction levels from interphase and mitotic cells. Several of the well-characterized mutated E2 proteins described above were used in this assay to enable us to correlate protein extraction with E2 function. These proteins were also used to further characterize the in vivo interaction and colocalization of E2 with Brd4. We demonstrate here that E2 stabilizes the interaction of Brd4 with chromatin during both interphase and mitosis; thus, the E2-Brd4 complex is not restricted to mitotic cells but is likely involved in transcriptional regulation by the E2 protein.
MATERIALS AND METHODS
Plasmids.
The pMEP E2 plasmid expression vector, from which wild-type and mutated E2 genes are expressed from an inducible metallothionein promoter, has previously been described (43). Detailed descriptions of the generation of the mutated E2 proteins pMEP E2-A1, pMEP E2-F3, and pMEP K342 are also available elsewhere (3, 54).
Cell culture.
The CV-1, C127, and 137 (35) cell lines were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. CV-1-derived lines were generated by transfection with the pMEP4 vector or an appropriate pMEP E2 plasmid by the use of Superfect transfection reagent (QIAGEN). Following selection in 200 μg/ml hygromycin, resistant colonies were pooled, expanded, and maintained in 200 μg/ml hygromycin for use in all experiments. P19 cells were maintained in alpha minimum essential medium containing ribonucleosides and deoxyribonucleosides and supplemented with 7.5% bovine calf serum and 2.5% fetal bovine serum.
TSA treatment.
Cells were grown on glass slides (Superfrost Plus) and blocked in S phase by the addition of 2 mM thymidine for 14 to 16 h. The thymidine block was released and the cells were cultured for 9 h, with trichostatin A (TSA) added to a final concentration of 50 ng/ml for the last 4 h.
Indirect immunofluorescence.
For analyses of interphase cells, cells were seeded directly onto glass slides (Superfrost Plus) and grown for 48 h. E2 expression was induced by the addition of 1.0 μM CdSO4 for 3 to 4 h prior to fixation. Mitotic cells were blocked in S phase by the addition of 2 mM thymidine for 14 to 16 h. The thymidine block was released and the cells were cultured for 9 h, with E2 expression induced by the addition of 1.0 μM CdSO4 for the last 3 to 4 h. The cells were either washed in cold phosphate-buffered saline (PBS) prior to fixation in 4% paraformaldehyde-PBS or fixed directly in 3.2% paraformaldehyde for 15 min at room temperature and then were permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. E2 was detected with the monoclonal antibody B201 used at a 1:10 dilution (provided by Elliot Androphy) and with goat anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC) (1:100 dilution; Jackson Immunochemicals). Brd4 was detected with a 1:250 dilution of the rabbit polyclonal antibody 2290 (11) and with goat anti-rabbit IgG conjugated to Texas Red or FITC (1:100 dilution; Jackson Immunochemicals). Brd4 preimmune serum was used at a dilution of 1:250 (provided by Keiko Ozato). Brd2 was detected with a rabbit polyclonal antibody at a dilution of 1:250 (10) and with goat anti-mouse IgG conjugated to FITC (1:100 dilution; Jackson Immunochemicals). Slides were mounted in Vectashield mounting medium (Vector Laboratories) containing 20 μg/ml of propidium iodide or 1 μg/ml of DAPI (4′,6′-diamidino-2-phenylindole). Immunofluorescent staining was detected and digital images were captured with a Leica TCS-SP2 laser scanning confocal imaging system.
Western blotting.
For each sample, 10 μg of total protein was heated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer at 100°C for 5 min. For detection of the E2 protein, samples were separated by SDS-PAGE in a 12% polyacrylamide gel, while samples used for the detection of the Brd4 protein were separated in a 7.5% polyacrylamide gel, and then electrotransferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore). Western blotting was performed according to standard protocols with either the anti-E2 antibody B201 (provided by Elliot Androphy) or an anti-Brd4 antibody (MCB2, which is a rabbit polyclonal antibody produced against a 14-amino-acid synthetic peptide corresponding to the C terminus of Brd4 [11]) followed by horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG, respectively (Pierce). Protein bands were detected by using the chemiluminescence reagent SuperSignal West Dura (Pierce). When necessary, membranes were stripped with 62.5 mM Tris-HCl, pH 6.7, 2% (vol/vol) SDS, 0.288 mM 2-mercaptoethanol at 50°C for 30 min and then reprobed with an antibody directed against lamin A/C (sc-6215; Santa Cruz Biotechnology, Inc.) followed by horseradish peroxidase-conjugated mouse anti-goat IgG (Pierce).
Protein extraction.
For analysis by immunofluorescence, slides were submerged in ice-cold CSK extraction buffer (10 mM PIPES, pH 6.8, 30 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100, Complete protease inhibitor [Roche]) (5) containing 300 mM NaCl and chilled on ice for 15 min, followed by fixation in 3.2% paraformaldehyde-PBS for 15 min. Unextracted slides were fixed directly in 3.2% paraformaldehyde-PBS for 15 min, as described above.
For Western blot analysis, cell monolayers were covered with ice-cold CSK extraction buffer (10 mM PIPES, pH 6.8, 30 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100, Complete protease inhibitor [Roche]) (5) containing 300 mM NaCl and chilled on ice for 15 min. Following removal of the salt solution, and for untreated samples, proteins were extracted in 50 mM Tris-HCl, pH 6.8, 2% (vol/vol) SDS, 10% glycerol, Complete protease inhibitor (Roche). The total protein concentrations in the extracts were determined by use of a bicinchoninic acid protein assay kit (Pierce).
RESULTS
Colocalization of E2 and Brd4 during mitosis.
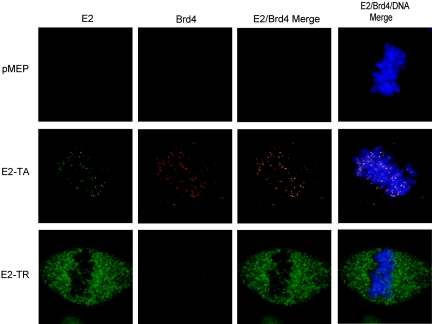
Brd4 was recently identified as a chromosome-bound cellular factor with which E2-TA interacts to mediate viral genome segregation (56). You et al. reported that E2 and Brd4 colocalize in punctate dots on mitotic chromosomes in C33A cells (56). For our study, colocalization of the Brd4 and E2 proteins was examined in CV-1 cells expressing the full-length transactivator protein, E2-TA, or the truncated repressor protein, E2-TR. In agreement with You et al., we found E2-TA to completely colocalize with Brd4 in distinct speckles randomly distributed over the chromosomes (Fig. 1). However, no colocalization with Brd4 was evident in mitotic cells that expressed E2-TR, which is unable to bind mitotic chromosomes. Brd4 could not be detected as distinct speckles on condensed chromosomes (Fig. 1), and only a faint diffuse coating could be observed. Moreover, this speckled pattern of chromosomal Brd4 staining was not detected in mitotic cells expressing the empty pMEP4 vector (Fig. 1) or in untransfected CV-1 cells in either the presence or absence of cadmium sulfate induction (data not shown). The only exceptions to this observation were cells in the early stages of prophase and the late stages of telophase; these cells were occasionally more positive for diffuse chromosomal Brd4 staining, presumably because they were transitioning from and to interphase, during which Brd4 levels are high.
FIG. 1.
E2 and Brd4 colocalize on mitotic chromosomes. The colocalization of E2 and Brd4 on mitotic chromosomes was examined in CV-1 cells expressing no E2 (pMEP), E2-TA, or E2-TR. The E2 protein, as detected by a FITC-labeled antibody, is shown in green; the Brd4 protein, as detected by a Texas Red-labeled antibody, is shown in red; and the colocalization of these proteins appears yellow in the merged images. Cellular DNA was detected by DAPI staining and is shown in blue.
Colocalization of E2-TA with Brd4 in cells transformed with the BPV-1 genome.
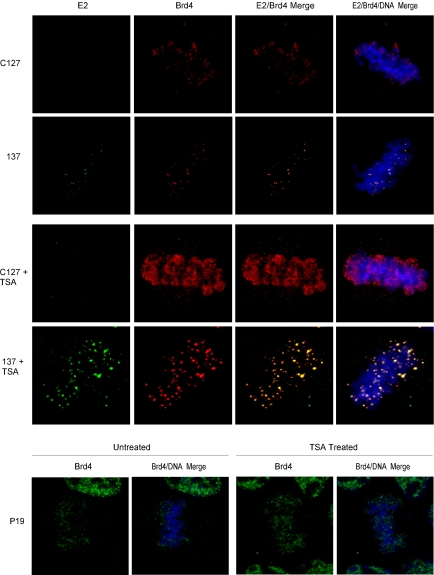
To confirm the relocalization and stabilization of Brd4 by E2 in cells expressing E2 from the episomal BPV genome and to establish that it was not specific to the CV-1 line, the localization of the E2 and Brd4 proteins was also examined in C127 cells transformed with BPV-1. The 137 cell line was established from C127 cells transformed with a high-copy-number BPV-1 genome that contains mutations in the major phosphorylation sites of the E2 protein; these mutations result in higher levels of E2 protein expression because of an increased protein half-life (35, 42, 43). In mitotic C127 cells, Brd4 was found to diffusely coat the condensed chromosomes at all stages of mitosis (Fig. 2, top row). This pattern is in agreement with the original observations of Dey et al. (11). However, in 137 cells, which are positive for E2 protein expression, Brd4 was observed in distinct speckles on mitotic chromosomes and colocalized with E2 throughout mitosis (Fig. 2, second row). Thus, despite differences in the levels of mitotic staining of Brd4 in CV-1 and C127 cells that did not express the E2 protein, the expression of E2 from either an extrachromosomal plasmid or an extrachromosomally replicating viral genome resulted in relocalization and/or stabilization of the mitotic association of Brd4 with chromosomes in these cell lines.
FIG. 2.
TSA increases the amount of chromosomally bound Brd4 in C127, 137, and P19 cells. (Top rows) E2 and Brd4 protein expression was compared by indirect immunofluorescence in C127 and 137 cells, with or without TSA treatment. The E2 protein, as detected by a FITC-labeled antibody, is shown in green; the Brd4 protein, as detected by a Texas Red-labeled antibody, is shown in red; and cellular DNA was detected by DAPI staining and is shown in blue. (Bottom row) The Brd4 protein in P19 cells, with or without TSA treatment, was detected by a FITC-labeled antibody and is shown in green. Cellular DNA was stained with DAPI and is shown in blue.
Brd4 has previously been shown to bind to acetylated histones, specifically H3 and H4 (10). The acetylation pattern of these histones changes during the cell cycle, such that the highly acetylated isoforms of H3 and H4 are lost during mitosis (24). C127 and 137 cells were treated with the histone deactylase inhibitor trichostatin A (TSA) to determine if an increase in the mitotic expression of these highly acetylated histones would alter the pattern of E2 and Brd4 colocalization observed in mitotic 137 cells. The treatment of C127 cells with TSA for 4 hours resulted in a dramatic increase in the level of chromosomal Brd4 staining at all mitotic stages (Fig. 2, third and fourth rows). Brd4 staining in TSA-treated C127 cells was diffuse and coated the chromosomes, similar to the case for untreated C127 cells. The treatment of 137 cells with TSA increased the intensity of the Brd4-E2 speckles and not the diffuse chromosomal coating of Brd4 (Fig. 2). Therefore, E2 is able to relocalize large amounts of the Brd4 protein, even on highly acetylated mitotic chromosomes.
The diffuse chromosomal staining pattern observed in C127 cells is in agreement with the original description of Brd4 staining in mitotic P19 cells reported by Dey et al. (11). We also examined the pattern of Brd4 expression in mitotic P19 cells and found that, as observed by Dey et al., Brd4 was distributed in a diffuse pattern over the chromosomes at all mitotic stages, and TSA treatment of these cells also resulted in an increase in the level of chromosome-bound Brd4 protein (Fig. 2, bottom row).
Transcriptionally competent E2 proteins colocalize with Brd4 on mitotic chromosomes.
A panel of mutated E2 proteins that are defective in specific E2 functions was previously described (3, 4). This panel has been characterized extensively for transcriptional regulatory and transient replication functions, interaction with the viral E1 protein, interaction with the Brd4 protein in vitro, and mitotic chromosome binding. Three key mutated E2 proteins with distinct functional defects were selected for a study of the interaction of E2 and Brd4 in vivo. The mutated A1 protein (containing the mutations E2A, E6A, E13A, and E20A) contains glutamate-to-alanine substitutions in the acidic residues of the amino-terminal amphipathic helix of the transactivation domain. This protein binds mitotic chromosomes, efficiently interacts with Brd4 in vitro, and regulates transcription similar to wild-type E2-TA (3). However, the transient replication activity of this protein is much reduced due to an inability to interact with the viral E1 protein (4). The F3 protein (containing the mutations R37A and I73A) does not bind to mitotic chromosomes or interact with Brd4, and it is defective for transcriptional regulation but retains a wild-type replication activity (3, 4). The DNA binding-defective protein E2 K342 contains an arginine-to-lysine substitution in the C-terminal DNA binding domain (54). Similar proteins defective in DNA binding retain the ability to bind to mitotic chromosomes because the transactivation domain is intact (50). However, this protein lacks transient replication activity and cannot regulate transcription since it can no longer bind to DNA (54). A list of the E2 proteins used for this study, together with a summary of their phenotypes, is shown in Table 1.
TABLE 1.
Phenotypes of E2 proteins used for this studya
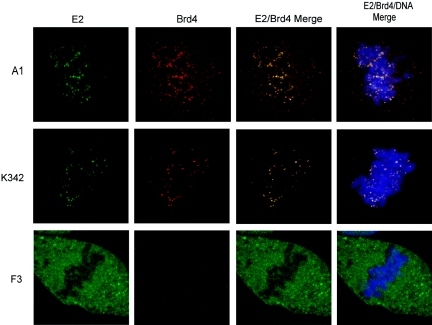
The colocalization of these mutated E2 proteins with Brd4 on mitotic chromosomes was examined. The A1 and K342 proteins were capable of binding mitotic chromosomes and colocalized with Brd4 in punctate dots distributed over the condensed chromosomes, similar to the observation made for E2-TA (Fig. 3). Therefore, despite its specific defects in DNA binding, the K342 protein colocalized with Brd4 on mitotic chromosomes, indicating that DNA binding is not required for this interaction. As shown previously, neither the mutated protein F3 nor the E2-TR protein bound mitotic chromosomes. Moreover, no punctate chromosomal Brd4 staining was detected in mitotic cells expressing either protein (Fig. 1 and 3). Thus, the expression of the E2-TA protein in CV-1 cells either stabilizes or relocalizes Brd4 to mitotic chromosomes. This colocalization is dependent on a transcriptionally competent E2 transactivation domain but does not require the DNA binding or replication function.
FIG. 3.
Chromosome-bound E2 proteins colocalize with Brd4 on mitotic chromosomes. The colocalization of E2 and Brd4 on mitotic chromosomes was examined in cells expressing the mutated E2 proteins A1, K342, and F3. The E2 protein, as detected by a FITC-labeled antibody, is shown in green; the Brd4 protein, as detected by a Texas Red-labeled antibody, is shown in red; and the colocalization of these proteins appears yellow in the merged images. Cellular DNA was detected by DAPI staining and is shown in blue.
E2 and Brd4 colocalize in interphase cells.
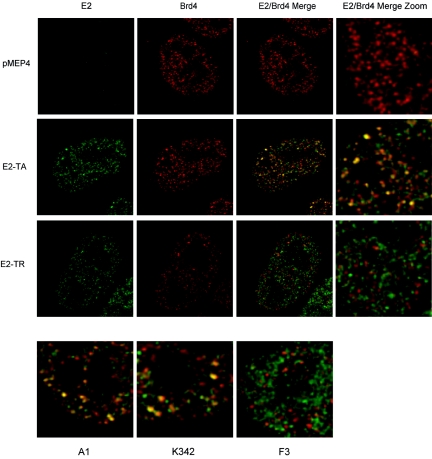
You et al. isolated an E2-Brd4 complex from an asynchronous cell population and found relatively large amounts of Brd4 in complex with E2 (56). Since the majority of these cells were in interphase, this indicates that E2 and Brd4 must also associate at different stages of the cell cycle. To examine this hypothesis, we analyzed the colocalization of Brd4 with E2 by indirect immunofluorescence. The colocalization of E2 and Brd4 was examined in interphase cells by the use of cell lines expressing E2-TA, E2-TR, and the mutated proteins A1, K342, and F3. Brd4 was abundantly expressed in the nuclei of interphase cells for all cell lines tested but was excluded from the nucleolar regions (Fig. 4). Figure 4 (top) shows representative images of CV-1 cells containing the empty pMEP4 vector or expressing either the E2-TA or E2-TR protein. The Brd4 protein was observed in a speckled pattern in each of these cells. There were no apparent differences in the patterns of Brd4 expression in the absence of E2 expression and in the presence of mutated E2 proteins. However, colocalization of the E2 and Brd4 proteins was only observed in cells expressing E2-TA and not E2-TR. Areas of colocalization appear yellow in the merged images of E2 and Brd4 shown in Fig. 4. In E2-TA cells, a subset of E2- and Brd4-containing speckles colocalized and overlapped, but distinct speckles containing each protein were observed that did not colocalize. Moreover, for cells expressing E2-TR, despite similar expression levels and individual patterns of the E2 and Brd4 proteins, very little colocalization was apparent and individual speckles of each protein were observed in the merged image. The mutated E2 proteins A1 and K342 also colocalized with Brd4, similar to the case for E2-TA (Fig. 4, bottom). In contrast, similar to the case for E2-TR, no colocalization was observed in cells expressing the mutated protein F3 (Fig. 4, bottom).
FIG. 4.
Colocalization of E2 and Brd4 in interphase cells. (Top) CV-1 cells expressing no E2 (pMEP4), E2-TA, or E2-TR were assayed for E2 and Brd4 localization by indirect immunofluorescence. (Bottom) Magnified images showing colocalization of Brd4 and mutated E2 proteins. The E2 protein, as detected by a FITC-labeled antibody, is shown in green; the Brd4 protein, as detected by a Texas Red-labeled antibody, is shown in red; and the colocalization of these proteins appears yellow in the merged images. Images were deconvolved by using Huygens Essential, version 2.6, software (SVI, Hilversum, The Netherlands).
The E2 proteins which colocalize with Brd4 in interphase cells (E2-TA, A1, and K342) share several common features: each is capable of associating with mitotic chromosomes, and each has an intact transactivation domain capable of regulating transcription. The mutated protein K342 cannot activate transcription in an E2 binding site-dependent manner because of its inability to bind DNA. However, the transactivation domain of this protein is intact and presumably remains capable of associating with a variety of cellular factors, as previously reported (8, 30, 41, 47, 55). Furthermore, this indicates that the colocalization of E2 and Brd4 in interphase cells is not dependent on active transcription. Active replication is also not important for the interphase association of these proteins, since the A1 and K342 proteins are both defective in replication yet colocalized with Brd4, while the mutated protein F3, which retains its replication activity, was unable to interact with this protein. Furthermore, the presence of an intact DNA binding domain is not sufficient for an E2-Brd4 interaction in vitro, since E2-TR showed no colocalization with Brd4. This is in agreement with the study of You et al., who showed that E2-TR could not interact with Brd4 in vitro (56). Therefore, based on the phenotypes of the mutated E2 proteins, we can state that the ability to colocalize with Brd4 in interphase cells correlates with the presence of a transactivation domain that is competent for transcriptional regulation, implicating the E2-Brd4 complex in transcriptional control.
The tight association of E2 and Brd4 with chromatin requires a transactivation domain that is competent for transcriptional regulation.
The colocalization of E2 and Brd4 in interphase cells indicates that this protein complex plays additional roles in the viral life cycle beyond simply tethering viral genomes to mitotic chromosomes. In interphase cells, E2 is known to function as a transcriptional regulator of viral genes, and in complex with the E1 protein, E2 can initiate viral replication. However, as shown above, colocalization of the E2 and Brd4 proteins during interphase appears to be dependent on the presence of a transactivation domain capable of regulating transcription. While the cellular function of Brd4 remains unknown, it was previously shown that Brd4 can be eluted from cells at salt concentrations of about 150 to 200 mM NaCl (10). The treatment of these cells with TSA prior to extraction resulted in a shift in peak elution to 600 mM NaCl, indicating that an increase in histone acetylation stabilizes the association of Brd4 with interphase nuclei (10).
The extraction of proteins under conditions of increasing salt can be used to assess protein binding to chromatin (10, 31, 48, 49). To more fully understand the interaction of E2 and Brd4 in interphase cells and the role of this interaction in the viral life cycle, we examined the extraction of both proteins with solutions at increasing salt concentrations. Cytoskeletal (CSK) extraction buffer was used for these studies. This hypotonic buffer is commonly used for the first step of nuclear matrix isolation to permeabilize cells and remove soluble proteins prior to chromatin digestion (reviewed in reference 6). We used CSK buffer containing 300 mM NaCl and examined the extraction of the E2 and Brd4 proteins under these conditions by indirect immunofluorescence and Western blotting.
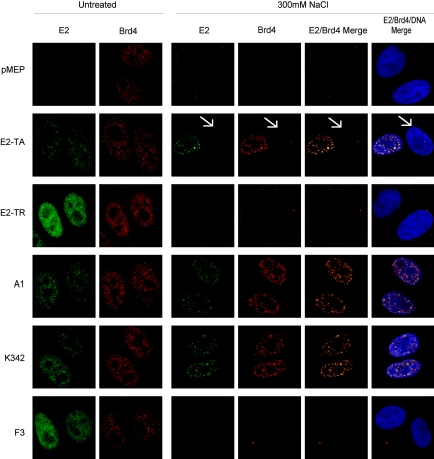
For indirect immunofluorescence, cells were grown directly on glass slides prior to extraction. Initially, extraction was assessed for the E2-TA transactivator and E2-TR repressor proteins. Before extraction, cells expressing either E2-TA or E2-TR showed distinct nuclear E2 staining (Fig. 5), while no E2 expression was detected in cells containing the empty pMEP4 vector (Fig. 5). The Brd4 protein was expressed in a speckled pattern in the nuclei of all cells, regardless of the expression of the E2 protein (Fig. 5). After extraction in 300 mM NaCl-CSK buffer, much of the E2-TA protein remained resistant to salt extraction (Fig. 5). However, E2-TR was completely removed under these conditions, with little or no remaining protein detected, indicating that this protein is not tightly bound to chromatin in interphase cells (Fig. 5). Moreover, the extraction of Brd4 from these cells differed also, such that Brd4 was retained only in cells that also expressed E2-TA (Fig. 5). Cells expressing either the empty pMEP vector or E2-TR protein showed little or no nuclear retention of the Brd4 protein following extraction (Fig. 5). Furthermore, the retention of Brd4 was completely dependent on the retention of E2, since individual cells from the E2-TA-expressing cell line that did not express the E2 protein did not show any retention of Brd4 (Fig. 5, E2-TA row, cell identified by arrow). In addition, the majority of E2 and Brd4 protein remaining in these cells following extraction colocalized in distinct speckles (Fig. 5). As a control, CV-1 cells or cells containing the empty pMEP vector were treated with the histone deacetylase inhibitor TSA to increase histone acetylation. This resulted in the nuclear retention of Brd4 in some, but not all, cells in 300 mM NaCl (data not shown). This stabilization of Brd4 with TSA is in agreement with previous observations by Dey et al. (10). Furthermore, it indicates that E2 can also stabilize the interaction of Brd4 with chromatin in interphase cells under the same conditions.
FIG. 5.
The association of E2 and Brd4 with interphase chromatin requires a transcriptionally competent transactivation domain. The E2 protein was detected by a FITC-labeled antibody (green), Brd4 expression was detected by a Texas Red-labeled antibody (red), and cellular DNA was detected by DAPI staining (blue). The panels on the left show untreated cells and those on the right show cells extracted in 300 mM NaCl.
The striking differences in behavior of the two naturally occurring forms of the E2 protein indicated that this procedure could be used to correlate the chromatin/nuclear matrix association and Brd4 interaction with E2 function. Cells expressing the mutated proteins A1, K342, and F3 were analyzed by the same extraction procedure. Proteins A1 and K342 behaved like wild-type E2-TA in the extraction experiments, with a salt-resistant protein subpopulation retained following extraction in 300 mM NaCl-CSK extraction buffer (Fig. 5). In contrast, the mutated protein F3 was easily extracted, with little or no protein detected following extraction with 300 mM NaCl-CSK extraction buffer, similar to the case for E2-TR (Fig. 5). The nuclear retention of the Brd4 protein correlated with that of the E2 proteins; Brd4 was retained only in cells which also retained either A1 or K342 and was not detected in cells expressing F3, which itself was also completely extracted under these conditions (Fig. 5).
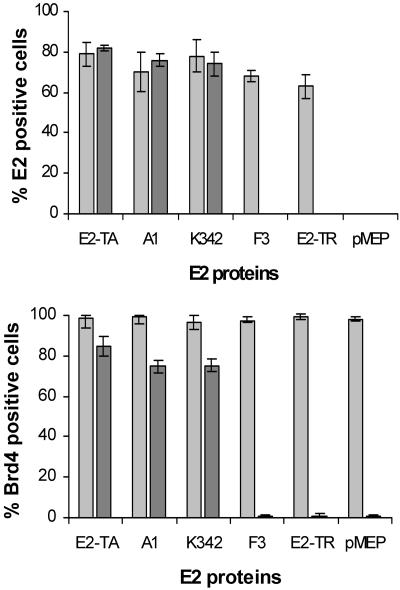
To quantitate these observations, we scored approximately 150 cells of each cell line for E2 and Brd4 protein expression before and after salt extraction. These results are shown in Fig. 6. For the E2-TA-, A1-, and K342-expressing cell lines, the number of cells positive for E2 protein expression did not change significantly following treatment, demonstrating that these proteins can interact tightly with the interphase nucleus. However, the E2-TR and F3 E2 proteins could not be detected after salt extraction, indicating that they do not associate tightly with nuclear chromatin. The Brd4 protein was detected in almost 100% of cells expressing each of the E2 proteins prior to extraction. However, following extraction, nuclear Brd4 protein was detected only in cells that also expressed the E2-TA, A1, or K342 protein. The number of cells positive for E2 and Brd4 protein expression following salt treatment correlated well, indicating that in the presence of these E2 proteins, the chromatin association of Brd4 is stabilized (Fig. 6).
FIG. 6.
Slides of each cell line were scored for E2 (top) and Brd4 (bottom) protein expression before and after salt extraction (light and dark gray bars, respectively). The number of cells that were positive for the E2 or Brd4 protein was expressed as a percentage of the total number of cells counted.
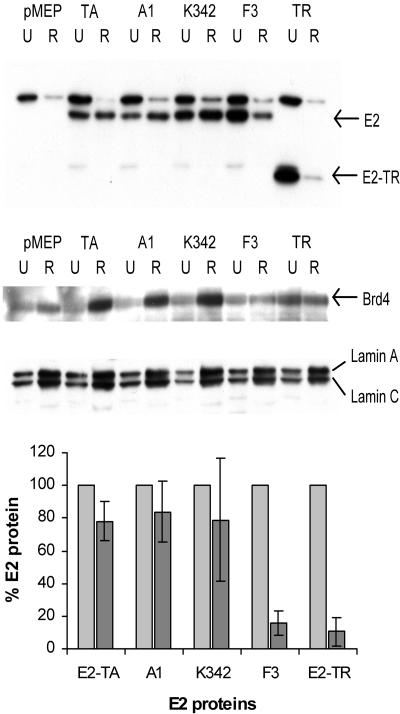
The retention of the E2 and Brd4 proteins was also examined by Western blot analysis. Equivalent amounts of protein lysates prepared from untreated cell monolayers or cells treated with 300 mM NaCl in extraction buffer were separated by SDS-PAGE and immunoblotted with either the anti-E2 antibody B201 or the anti-Brd4 antibody MCB2. The retention of E2 and Brd4 proteins following extraction can be compared for each cell line in the representative Western blots shown in Fig. 7. The cellular protein lamin A/C was used as an internal loading control. Lamin A/C is an integral component of the nuclear envelope which associates with chromatin and binds matrix attachment regions and thus is retained under these extraction conditions (13, 39). Equal amounts of total protein lysates were analyzed, and thus the enriched chromatin/nuclear matrix-associated proteins are overrepresented relative to the unextracted protein samples. A quantitative analysis of four experiments indicated that at least 75% of E2-TA and the mutated proteins A1 and K342 was retained after extraction, whereas the amount of E2 protein detected following treatment of the E2-TR- and F3-expressing cell lines was <16% (Fig. 7, bottom panel). The increased retention of the Brd4 protein correlated with that of the E2 proteins. With cells expressing the E2-TA, A1, and K342 proteins, we saw, on average, a threefold increase in retention of the Brd4 protein after extraction, indicating that in the presence of these E2 proteins, almost all of the cellular Brd4 protein becomes tightly associated with chromatin. However, in cells expressing either E2-TR or the F3 protein, the Brd4 protein was not retained more than that in cells that do not contain E2 protein, demonstrating that these E2 proteins could not stabilize the association of Brd4 with nuclear chromatin (Fig. 7).
FIG. 7.
Retention of E2 and Brd4 proteins requires a transactivation domain capable of transcriptional regulation. (Top) Western blots of protein extracts from CV-1 cell lines expressing E2 proteins before (U) and after (R) salt extraction. The E2 proteins were detected with the anti-E2 antibody B201 (top blot). The Brd4 protein was detected with the anti-Brd4 antibody MCB2 and is marked by an arrow (middle blot). The membranes were stripped and reprobed with an anti-lamin A/C antibody, sc-6215 (bottom blot). Lamin A runs at approximately 70 kDa, while lamin C runs at 65 kDa. (Bottom) Quantitation of E2 protein retention. The data are averages from four experiments. The amount of E2 protein retained (dark gray bars) is expressed relative to the amount of E2 protein in an untreated sample (light gray bars) of each cell line, which was set to 100%.
Almost all of the total Brd4 protein was retained/stabilized in cells expressing E2, whereas about one-third of the E2 protein was associated with the chromatin/nuclear matrix fraction after taking into account the enrichment of proteins in the extracted lysates. This was probably because Brd4 was endogenously expressed in these cells while E2 was transiently induced from a heterologous promoter just 4 hours before extraction. Under these conditions, only a proportion of E2 might be localized to chromatin and/or the nuclear matrix. The tight association of E2 proteins with chromatin correlates with the presence of a transactivation domain that is competent for transcriptional regulation, suggesting that a functional interaction of E2 with the cellular transcriptional regulation machinery stabilizes its association with chromatin and/or the nuclear matrix and protects it from salt extraction. The A1 protein is specifically defective in transient replication activity but can stabilize Brd4 as well as wild-type E2, and therefore, the nuclear retention of Brd4 does not require the replication function of E2. The Brd4 protein was easily extracted from cells expressing either E2-TR or the mutated protein F3, both of which are defective for chromosome association and transcriptional regulation. Therefore, the colocalization of E2 and Brd4 in interphase cells, the increased nuclear retention of Brd4 in the presence of E2 protein, and the colocalization of these proteins after extraction require an E2 protein with a transactivation domain that is competent for transcriptional regulation. Thus, the E2-Brd4 complex must have additional roles in the viral life cycle, most likely through transcriptional control.
Brd2 is not resistant to salt extraction from interphase cells in the presence of E2.
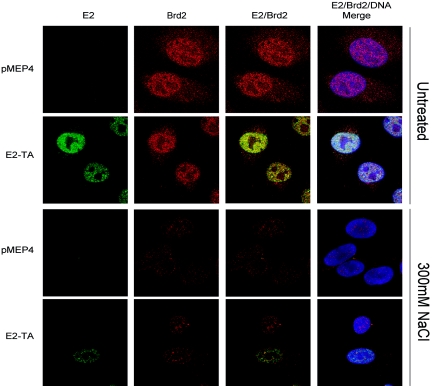
To establish that the retention of Brd4 following extraction in moderate salt was a result of a specific interaction with E2, we examined the extraction of another BET family member. Brd2 (formerly RING3) is structurally similar to Brd4 but lacks the C-terminal “tail” region and is unable to bind to E2 (3). The extraction of Brd2 was examined in control cells and cells expressing E2-TA. As shown in Fig. 8, Brd2 was predominantly detected in the nuclei of interphase cells in a speckled pattern in both cell lines. However, following extraction of these cells in 300 mM NaCl-CSK extraction buffer, Brd2 expression was greatly reduced, and this observation was similar in both control cells and cells expressing E2-TA (Fig. 8). There was no difference in the amounts of Brd2 protein remaining in individual cells from the E2-TA-expressing cell line that were positive or negative for E2 protein expression, indicating that E2 expression could not prevent the extraction of this cellular protein. Therefore, the retention of Brd4 in cells expressing E2 following salt extraction is specific and is most likely due to the previously described direct interaction between E2 and Brd4.
FIG. 8.
E2 expression does not stabilize the association of Brd2 with interphase nuclei. The E2 protein was detected by a FITC-labeled antibody and is shown in green, the Brd2 protein was detected by a Texas Red-labeled antibody and is shown in red, and cellular DNA was detected by DAPI staining and is shown in blue. Brd2 protein levels were compared between cells expressing no E2 (pMEP4) and cells expressing E2-TA. The top panels show untreated cells and the bottom panels show cells following extraction in 300 mM NaCl.
DISCUSSION
Chromatin modifications such as phosphorylation, methylation, and acetylation regulate changes in chromatin structure that are required for chromosome segregation, replication, and transcription. In particular, histone acetylation is pivotal to the regulation of gene expression (reviewed in references 38 and 52), with the most highly acetylated chromatin states being associated with transcriptional competency. As cells progress through the cell cycle into mitosis, a striking decrease in transcriptional activity is observed, coincident with mitotic condensation of the chromosomes (reviewed in reference 14). Chromosome condensation reduces the accessibility of the transcriptional machinery to target sites, and many chromatin-associated transcription regulatory proteins dissociate from the DNA during mitosis (32). The global pattern of histone acetylation is also dramatically altered as cells pass into mitosis; the highly acetylated core histones H3 and H4 are barely detectable in metaphase, anaphase, and early telophase and only become visible again as chromosome decondensation begins in late mitosis (24). This reappearance of acetylated histones coincides with the resumption of transcription in late telophase (14).
The bromodomain protein Brd4 is unusual in that it remains associated with chromatin throughout mitosis (hence the original designation of Brd4 was mitotic chromosome-associated protein) (10, 11). In this study, we show that the endogenous pattern of Brd4 expression on mitotic chromosomes is variable among cell lines. We found that, as first shown by Dey et al., the Brd4 protein forms a diffuse coat over mitotic chromosomes in human P19 embryonal carcinoma cells, mouse 3T3 fibroblasts (11), and C127 cells. However, You et al. reported that Brd4 forms punctate dots on the condensed mitotic chromosomes of human C33A cells, even in the absence of the E2 protein (56). We found that a diffuse chromosomal coat of Brd4 was detectable at all mitotic stages in C127 cells, while in monkey CV-1 cells, the Brd4 protein was detected in prophase, barely detectable in metaphase and anaphase, and by telophase again observed associated with condensed chromatin, consistent with the resumption of transcription (14) and de novo expression (23).
Brd4 binds to mitotic chromosomes via acetylated histones, and any change in acetylation is predicted to affect Brd4 binding (10). In support of this, we showed that chromosomal histone acetylation and Brd4 staining were dramatically increased during all mitotic stages in C127 cells by a treatment with TSA (Fig. 2, top panel), as shown originally by Dey et al. for other cell types. Therefore, the observed decrease in chromosomal Brd4 staining in many cells was most likely due to decreased histone acetylation during mitosis.
Despite the differences in the patterns of mitotic Brd4 expression in different cell types in the absence of the E2 protein, the pattern observed in the presence of E2 was highly consistent. E2 and Brd4 colocalize specifically in distinct speckles on mitotic chromosomes in the human cervical carcinoma line C33A (56; J. Oliveira and A. A. McBride, unpublished observations) and, as shown here, in monkey CV-1 cells and mouse C127 cells (Fig. 1, 2, and 3). In the experiments presented here, E2 expression resulted in relocalization and/or stabilization of the Brd4 protein into punctate complexes on the mitotic chromosomes.
There are several potential mechanisms by which E2 could stabilize and/or relocalize Brd4. Since the chromosomal association of Brd4 is dependent on histone acetylation, the E2 protein could increase Brd4 binding by modulating the expression or activity of histone acetyltransferases (HATs) or deacetylases. The former model seems unlikely because we showed that the DNA binding function of E2 is not required for the stabilization of Brd4 on chromatin. In support of the latter model, E2 is known to directly interact with HAT proteins such as p300/CBP and pCAF (27, 40, 41). Another possibility is that the Brd4-E2 complex has an altered specificity for chromatin that enables it to bind to hypoacetylated mitotic chromosomes. Alternatively, the levels of histone acetylation may not be altered, but rather a diffuse and almost undetectable chromosomal coating of Brd4 could be coalesced into distinct chromosomal regions by the E2 protein. There is a precedent for the last mechanism in that LANA-1, the analogous viral tethering protein for human herpesvirus 8 (HHV-8), interacts with another BET family member, Brd2 (34, 46). Brd2 also shows variable mitotic chromosomal associations (10, 34), but the expression of LANA stabilizes and relocalizes the Brd2 protein to regions of heterochromatin (34, 46).
Studies of chromatin association and mobility have shown that Brd4 is highly mobile within the nucleus and associates transiently with chromatin (10, 44). This mobility is reduced by the treatment of cells with TSA, which increases histone acetylation and stabilizes the association of Brd4 with chromatin (10). We showed here that E2 proteins that are transcriptionally competent also stabilize the association of Brd4 with chromatin.
Only the E2 proteins E2-TA, A1, and K342, which have intact transactivation domains that are capable of transcriptional activation, were themselves tightly associated with the nucleus in interphase cells. These E2 proteins also colocalized with Brd4, partially in interphase cells and completely during mitosis, and stabilized its association with chromatin. Conversely, E2-TR and the F3 protein, which are defective for transcriptional regulation, did not colocalize with Brd4, were themselves easily eluted from cells, did not bind mitotic chromosomes, and could not stabilize the interaction of Brd4 with chromatin. Therefore, the colocalization and retention of E2 and Brd4 proteins in the nucleus correlate with the transcription regulation capability of E2. Furthermore, these results indicate that the E2-Brd4 interaction occurs during interphase as well as during mitosis and likely has additional roles in the viral life cycle related to E2's function as a transcriptional regulator.
Several members of the BET protein family, including Drosophila melanogaster Fsh, yeast Bdf1, and human Brd2, function as transcriptional regulators (12). Moreover, Brd4 is associated with the mouse mediator of transcriptional regulation, suggesting that Brd4 too may play a role in transcription (18, 22). E2 interacts with the chromatin modifiers p300/CBP and pCAF (27, 40, 41). These bromodomain proteins possess intrinsic histone acetyltransferase (HAT) activity, which is also required for their transcriptional coactivator activities. E2 also binds components of the basal transcription machinery, including Sp1, TFIIB, and TATA-binding protein (TBP) (30, 47, 51, 55). Therefore, the interaction of E2 and Brd4, together with their associated proteins, may provide a functional link between chromatin remodeling and transcriptional regulation.
An analysis of the E2 proteins K342 and E2-TR demonstrated that the ability of E2 to bind DNA is neither required nor sufficient for the colocalization and stabilization of E2 and Brd4 in interphase or mitosis. Furthermore, active transcription per se is not required for the interaction and chromatin association of E2 and Brd4 in interphase cells, since the mutated protein K342 is unable to transactivate due to a defect in DNA binding yet colocalizes with Brd4 both during interphase and on mitotic chromosomes.
Both BPV-1 and human papillomavirus 11 E2 proteins have been shown previously to associate with the detergent-insoluble nuclear matrix (19, 57). In the latter case, tightly nucleus-associated E2 was found in replication foci in interphase cells that also contained E1 as well as the cellular replication factor replication protein A (53). In our study, replication-competent but transcriptionally defective E2 proteins were not tightly associated in the nucleus (F3 protein), whereas the replication-defective A1 protein behaved like wild-type E2. However, neither the viral E1 protein nor a viral replicon was present in our experiments, and the presence of these factors as well as the active replication process may recruit E2 to replication foci and result in a tighter association with S-phase nuclei.
The association of Brd4 with chromatin seems to be a somewhat labile interaction that is modulated by histone acetylation. This association does not seem sufficient to function as the stable tether that papillomaviruses need to ensure the transmission of their genomes to daughter cells. Thus, the stabilization of this interaction by the E2 protein is necessary for it to function as a stable tether for viral genomes. Thus, E2 is not simply a passive hitchhiker tagging along with a convenient cellular protein to successfully segregate viral genomes. The role of E2 in this association with Brd4 is much more active than was previously assumed, such that E2 might be better described as a hijacker, which changes the behavior of a cellular protein to its own end.
Acknowledgments
We thank Jaquelline Oliveira and Carl Baker for critical comments on the manuscript and Anup Dey for useful discussions.
REFERENCES
- 1.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 2.Bastien, N., and A. A. McBride. 2000. Interaction of the papillomavirus E2 with mitotic chromosomes. Virology 270:124-134. [DOI] [PubMed] [Google Scholar]
- 3.Baxter, M. K., M. G. McPhillips, K. Ozato, and A. A. McBride. 2005. The mitotic chromosome binding activity of the papillomavirus E2 protein correlates with interaction with the cellular chromosomal protein, Brd4. J. Virol. 79:4806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter, M. K., and A. A. McBride. 2005. An acidic amphipathic helix in the bovine papillomavirus E2 protein is critical for DNA replication and interaction with the E1 protein. Virology 332:78-88. [DOI] [PubMed] [Google Scholar]
- 5.Berezney, R., and D. S. Coffey. 1974. Identification of a nuclear protein matrix. Biochem. Biophys. Res. Commun. 60:1410-1417. [DOI] [PubMed] [Google Scholar]
- 6.Berezney, R., M. J. Mortillaro, H. Ma, X. Wei, and J. Samarabandu. 1995. The nuclear matrix: a structural milieu for genomic function. Int. Rev. Cytol. 162A:1-65. [DOI] [PubMed] [Google Scholar]
- 7.Brannon, A. R., J. A. Maresca, J. D. Boeke, M. A. Basrai, and A. A. McBride. 2005. Reconstitution of papillomavirus E2-mediated plasmid maintenance in Saccharomyces cerevisiae by the Brd4 bromodomain protein. Proc. Natl. Acad. Sci. USA 102:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breiding, D. E., F. Sverdrup, M. J. Grossel, N. Moscufo, W. Boonchai, and E. J. Androphy. 1997. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Mol. Cell. Biol. 17:7208-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe, J., P. Vaillancourt, A. Stenlund, and M. Botchan. 1989. Bovine papillomavirus type 1 encodes two forms of a transcriptional repressor: structural and functional analysis of new viral cDNAs. J. Virol. 63:1743-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey, A., F. Chitsaz, A. Abbasi, T. Misteli, and K. Ozato. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100:8758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey, A., J. Ellenberg, A. Farina, A. E. Coleman, T. Maruyama, S. Sciortino, J. Lippincott-Schwartz, and K. Ozato. 2000. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. Mol. Cell. Biol. 20:6537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florence, B., and D. V. Faller. 2001. You bet-cha: a novel family of transcriptional regulators. Front. Biosci. 6:D1008-D1018. [DOI] [PubMed] [Google Scholar]
- 13.Goldman, R. D., Y. Gruenbaum, R. D. Moir, D. K. Shumaker, and T. P. Spann. 2002. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16:533-547. [DOI] [PubMed] [Google Scholar]
- 14.Gottesfeld, J. M., and D. J. Forbes. 1997. Mitotic repression of the transcriptional machinery. Trends Biochem. Sci. 22:197-202. [DOI] [PubMed] [Google Scholar]
- 15.Grogan, E. A., W. P. Summers, S. Dowling, D. Shedd, L. Gradoville, and G. Miller. 1983. Two Epstein-Barr viral nuclear neoantigens distinguished by gene transfer, serology, and chromosome binding. Proc. Natl. Acad. Sci. USA 80:7650-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, A., B. D. Young, and B. E. Griffin. 1985. Random association of Epstein-Barr virus genomes with host cell metaphase chromosomes in Burkitt's lymphoma-derived cell lines. J. Virol. 56:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes, S. R., C. Dollard, F. Winston, S. Beck, J. Trowsdale, and I. B. Dawid. 1992. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucleic Acids Res. 20:2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houzelstein, D., S. L. Bullock, D. E. Lynch, E. F. Grigorieva, V. A. Wilson, and R. S. Beddington. 2002. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22:3794-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbert, N. L., J. T. Schiller, D. R. Lowy, and E. J. Androphy. 1988. Bovine papilloma virus-transformed cells contain multiple E2 proteins. Proc. Natl. Acad. Sci. USA 85:5864-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilves, I., S. Kivi, and M. Ustav. 1999. Long-term episomal maintenance of bovine papillomavirus type 1 plasmids is determined by attachment to host chromosomes, which is mediated by the viral E2 protein and its binding sites. J. Virol. 73:4404-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeanmougin, F., J. M. Wurtz, B. Le Douarin, P. Chambon, and R. Losson. 1997. The bromodomain revisited. Trends Biochem. Sci. 22:151-153. (Letter.) [DOI] [PubMed] [Google Scholar]
- 22.Jiang, Y. W., P. Veschambre, H. Erdjument-Bromage, P. Tempst, J. W. Conaway, R. C. Conaway, and R. D. Kornberg. 1998. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc. Natl. Acad. Sci. USA 95:8538-8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs, J. E., C. J. Fry, M. L. Samuels, and C. L. Peterson. 2000. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102:587-598. [DOI] [PubMed] [Google Scholar]
- 24.Kruhlak, M. J., M. J. Hendzel, W. Fischle, N. R. Bertos, S. Hameed, X. J. Yang, E. Verdin, and D. P. Bazett-Jones. 2001. Regulation of global acetylation in mitosis through loss of histone acetyltransferases and deacetylases from chromatin. J. Biol. Chem. 276:38307-38319. [DOI] [PubMed] [Google Scholar]
- 25.Lambert, P. F., B. A. Spalholz, and P. M. Howley. 1987. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell 50:69-78. [DOI] [PubMed] [Google Scholar]
- 26.Law, M. F., D. R. Lowy, I. Dvoretzky, and P. M. Howley. 1981. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc. Natl. Acad. Sci. USA 78:2727-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, D., S. G. Hwang, J. Kim, and J. Choe. 2002. Functional interaction between p/CAF and human papillomavirus E2 protein. J. Biol. Chem. 277:6483-6489. [DOI] [PubMed] [Google Scholar]
- 28.Lee, D., B. Lee, J. Kim, D. W. Kim, and J. Choe. 2000. cAMP response element-binding protein-binding protein binds to human papillomavirus E2 protein and activates E2-dependent transcription. J. Biol. Chem. 275:7045-7051. [DOI] [PubMed] [Google Scholar]
- 29.Lehman, C. W., and M. R. Botchan. 1998. Segregation of viral plasmids depends on tethering to chromosomes and is regulated by phosphorylation. Proc. Natl. Acad. Sci. USA 95:4338-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, R., J. D. Knight, S. P. Jackson, R. Tjian, and M. R. Botchan. 1991. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell 65:493-505. [DOI] [PubMed] [Google Scholar]
- 31.Lichota, J., and K. D. Grasser. 2001. Differential chromatin association and nucleosome binding of the maize HMGA, HMGB, and SSRP1 proteins. Biochemistry 40:7860-7867. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Balbas, M. A., A. Dey, S. K. Rabindran, K. Ozato, and C. Wu. 1995. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell 83:29-38. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama, T., A. Farina, A. Dey, J. Cheong, V. P. Bermudez, T. Tamura, S. Sciortino, J. Shuman, J. Hurwitz, and K. Ozato. 2002. A mammalian bromodomain protein, Brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell. Biol. 22:6509-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattsson, K., C. Kiss, G. M. Platt, G. R. Simpson, E. Kashuba, G. Klein, T. F. Schulz, and L. Szekely. 2002. Latent nuclear antigen of Kaposi's sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J. Gen. Virol. 83:179-188. [DOI] [PubMed] [Google Scholar]
- 35.McBride, A. A., and P. M. Howley. 1991. Bovine papillomavirus with a mutation in the E2 serine 301 phosphorylation site replicates at a high copy number. J. Virol. 65:6528-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBride, A. A., and G. Myers. 1997. The E2 proteins: an update, p. III54-III99. .In G. Myers, C. Baker, K. Munger, F. Sverdrup, A. McBride, and H.-U. Bernard (ed.), Human papillomaviruses 1997. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 37.McBride, A. A., H. R. Romanczuk, and P. M. Howley. 1991. The papillomavirus E2 regulatory proteins. J. Biol. Chem. 266:18411-18414. [PubMed] [Google Scholar]
- 38.Mizzen, C. A., and C. D. Allis. 1998. Linking histone acetylation to transcriptional regulation. Cell Mol. Life Sci. 54:6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moir, R. D., T. P. Spann, and R. D. Goldman. 1995. The dynamic properties and possible functions of nuclear lamins. Int. Rev. Cytol. 162B:141-182. [DOI] [PubMed] [Google Scholar]
- 40.Muller, A., A. Ritzkowsky, and G. Steger. 2002. Cooperative activation of human papillomavirus type 8 gene expression by the E2 protein and the cellular coactivator p300. J. Virol. 76:11042-11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng, Y. C., D. E. Breiding, F. Sverdrup, J. Richard, and E. J. Androphy. 2000. AMF-1/Gps2 binds p300 and enhances its interaction with papillomavirus E2 proteins. J. Virol. 74:5872-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penrose, K. J., M. Garcia-Alai, G. de Prat-Gay, and A. A. McBride. 2004. Casein kinase II phosphorylation-induced conformational switch triggers degradation of the papillomavirus E2 protein. J. Biol. Chem. 279:22430-22439. [DOI] [PubMed] [Google Scholar]
- 43.Penrose, K. J., and A. A. McBride. 2000. Proteasome-mediated degradation of the papillomavirus E2-TA protein is regulated by phosphorylation and can modulate viral genome copy number. J. Virol. 74:6031-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phair, R. D., P. Scaffidi, C. Elbi, J. Vecerova, A. Dey, K. Ozato, D. T. Brown, G. Hager, M. Bustin, and T. Misteli. 2004. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol. Cell. Biol. 24:6393-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piirsoo, M., E. Ustav, T. Mandel, A. Stenlund, and M. Ustav. 1996. Cis and trans requirements for stable episomal maintenance of the BPV-1 replicator. EMBO J. 15:1-11. [PMC free article] [PubMed] [Google Scholar]
- 46.Platt, G. M., G. R. Simpson, S. Mittnacht, and T. F. Schulz. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73:9789-9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rank, N. M., and P. F. Lambert. 1995. Bovine papillomavirus type 1 E2 transcriptional regulators directly bind two cellular transcription factors, TFIID and TFIIB. J. Virol. 69:6323-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sears, J., J. Kolman, G. M. Wahl, and A. Aiyar. 2003. Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by Epstein-Barr nuclear antigen 1. J. Virol. 77:11767-11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiio, Y., R. N. Eisenman, E. C. Yi, S. Donohoe, D. R. Goodlett, and R. Aebersold. 2003. Quantitative proteomic analysis of chromatin-associated factors. J. Am. Soc. Mass Spectrom. 14:696-703. [DOI] [PubMed] [Google Scholar]
- 50.Skiadopoulos, M. H., and A. A. McBride. 1998. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J. Virol. 72:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steger, G., J. Ham, O. Lefebvre, and M. Yaniv. 1995. The bovine papillomavirus 1 E2 protein contains two activation domains: one that interacts with TBP and another that functions after TBP binding. EMBO J. 14:329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Struhl, K. 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12:599-606. [DOI] [PubMed] [Google Scholar]
- 53.Swindle, C. S., N. Zou, B. A. Van Tine, G. M. Shaw, J. A. Engler, and L. T. Chow. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winokur, P. L., and A. A. McBride. 1996. The transactivation and DNA binding domains of the BPV-1 E2 protein have different roles in cooperative origin binding with the E1 protein. Virology 221:44-53. [DOI] [PubMed] [Google Scholar]
- 55.Yao, J. M., D. E. Breiding, and E. J. Androphy. 1998. Functional interaction of the bovine papillomavirus E2 transactivation domain with TFIIB. J. Virol. 72:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You, J., J. L. Croyle, A. Nishimura, K. Ozato, and P. M. Howley. 2004. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117:349-360. [DOI] [PubMed] [Google Scholar]
- 57.Zou, N., B. Y. Lin, F. Duan, K. Y. Lee, G. Jin, R. Guan, G. Yao, E. J. Lefkowitz, T. R. Broker, and L. T. Chow. 2000. The hinge of the human papillomavirus type 11 E2 protein contains major determinants for nuclear localization and nuclear matrix association. J. Virol. 74:3761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]