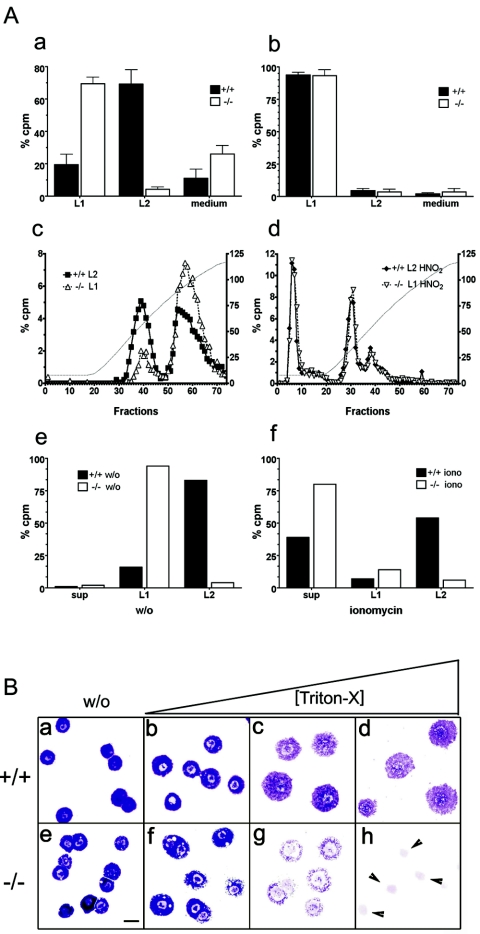
FIG. 6.
(A) Heparin biosynthesis and secretion in Mc-cpa+/+ and Mc-cpa−/− mast cells. Sorted peritoneal (a) or cultured BM-derived (b) mast cells were metabolically labeled by overnight culture in medium containing [35S]sulfate. Percent radioactivity in counts per minute (cpm) recovered in either the lysis fraction (L1) or salt extraction fraction (L2) is shown for Mc-cpa+/+ (+/+) (solid bars) and Mc-cpa−/− (−/−) (open bars) mast cells. Proteoglycans were further fractionated by ion-exchange chromatography and LiCl gradient elution (c). The left and right peaks correspond to chondroitin sulfate and heparin, respectively (10). The heparin peaks disappeared after treatment of the material with HNO2 prior to ion-exchange chromatography (d). The left y axes in panels c and d show percent counts per minute, and the right y axes show conductivity of the LiCl gradient in milli-Siemens per centimeter. Mast cells were pulse-chase-labeled with 35S. There was no spontaneous release into the supernatant (sup), but the material was retained in fractions L1 and L2 (e). w/o, without ionomycin. Following ionomycin (iono) stimulation, mast cells released labeled proteoglycans into the supernatant (sup) (f). (B) Mc-cpa−/− mast cells are highly detergent sensitive. Peritoneal mast cells from Mc-cpa+/+ (a to d) and Mc-cpa−/− (e to h) mice were cytocentrifuged in the absence (without [w/o]) (a and e) or presence of increasing amounts of Triton X-100(0.016% [b and f], 0.02% [c and g], and 0.5% [d and h]) and stained with toluidine blue. Wild-type mast cells withstood this treatment up to 0.5% Triton X-100 (d) but Mc-cpa−/− mast cells showed “holes” at low detergent concentrations (f) and disintegrated entirely at higher concentrations (g and h). The arrowheads in panel h point to the remaining nuclei. The scale bar in panel E (20 μm) applies to panels a to h.