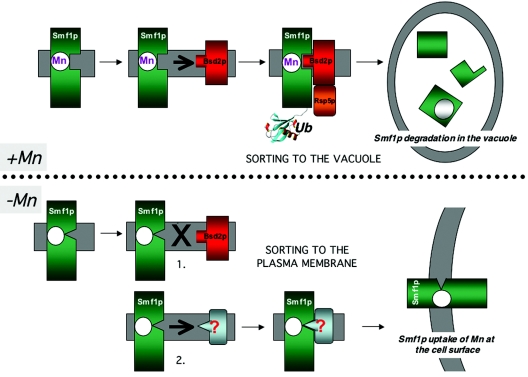
FIG. 2.
Manganese regulation of Smf1p at the level of protein sorting in the secretory pathway. Shown is a model depicting the way yeast cells may respond to changes in manganese availability by shifting localization of the Smf1p manganese transporter. (Top) When cells have ample manganese (e.g., grown in medium supplemented with >1 μM Mn [“+Mn”]), Smf1p in the secretory pathway is thought to interact with manganese and adopt a conformation that is recognized by membrane-bound Bsd2p. Bsd2p recruits the E3 ubiquitin ligase Rsp5, and Smf1p becomes tagged with ubiquitin (“Ub”), resulting in movement of Smf1p to the vacuole for degradation by vacuolar proteases. (Bottom) When cells are starved for manganese (extracellular manganese concentration of less than approximately 1.0 μM [“−Mn”]), Smf1p adopts a conformation that is not recognized by Bsd2p (step 1). This lack of recognition by Bsd2p is not sufficient to move Smf1p to the cell surface, and in a second step, manganese-free Smf1p may be recognized by another protein trafficking factor (as yet unknown; “?”) that helps direct Smf1p to the cell surface for the uptake of manganese from the growth medium.