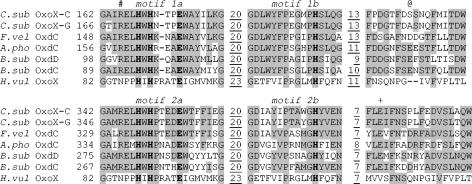
FIG. 2.
Protein motif sequence alignments of the C. subvermispora oxalate oxidase allelic isoforms with oxalate-degrading enzymes. The OxoX-C and OxoX-G allelic isoforms of C. subvermispora (C. sub) oxalate oxidase are aligned with the oxalate decarboxylases from F. velutipes (F. vel) (GenBank accession no. AAF13275), A. phoenices (A. pho) (GenBank accession no. AAE83943), and B. subtilis (B. sub) (OxdD GenBank accession no. O34767; OxdC GenBank accession no. O34714). The sequence of barley (H. vulgare [H. vul]) oxalate oxidase (GenBank accession no. P45850) is also shown for comparison, but this was structurally aligned with the B. subtilis OxdC C-terminal and N-terminal domains. Only the alignment of the duplicated cupin motifs (labeled in italics) together with regions downstream are shown. The first amino acid of each cupin motif is numbered, and the number of any intervening amino acids is indicated with underlining. The residues known to ligate mononuclear manganese ions in B. subtilis OxdC and barley OxoX are highlighted in boldface type. Conserved amino acids are highlighted with a grey background. Arg92 (B. subtilis OxdC sequence and numbering) is indicated by #, Glu162 is indicated by @, and Glu333 is indicated by +.