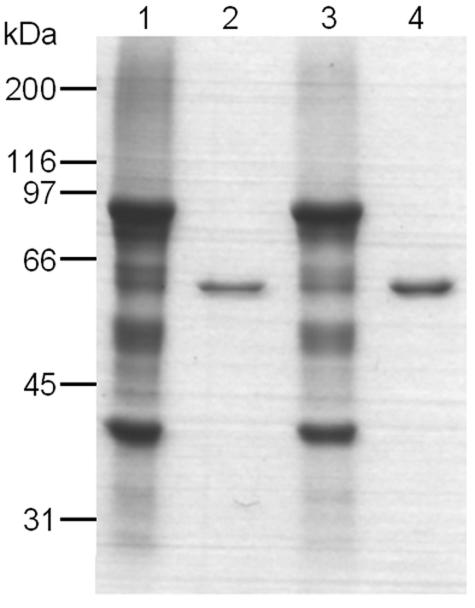
FIG. 8.
Coomassie brilliant blue R250-stained SDS-PAGE comparing the yields of the GST-toxin constructs and the stabilities of the mutant toxin constructs in the presence of trypsin. Two mutant toxin constructs, R521C and V582C, were solubilized and activated by trypsin treatment. The details are described in Materials and Methods. Solubilized GST-R521C (lane 1), trypsin-activated R521C (lane 2), solubilized GST-V582C (lane 3), and trypsin-activated V582C (lane 4) were subjected to SDS-PAGE. The proteins on the gel were visualized by Coomassie brilliant blue staining.