Abstract
The sea ice microbial community plays a key role in the productivity of the Southern Ocean. Exopolysaccharide (EPS) is a major component of the exopolymer secreted by many marine bacteria to enhance survival and is abundant in sea ice brine channels, but little is known about its function there. This study investigated the effects of temperature on EPS production in batch culture by CAM025, a marine bacterium isolated from sea ice sampled from the Southern Ocean. Previous studies have shown that CAM025 is a member of the genus Pseudoalteromonas and therefore belongs to a group found to be abundant in sea ice by culture-dependent and -independent techniques. Batch cultures were grown at −2°C, 10°C, and 20°C, and cell number, optical density, pH, glucose concentration, and viscosity were monitored. The yield of EPS at −2°C and 10°C was 30 times higher than at 20°C, which is the optimum growth temperature for many psychrotolerant strains. EPS may have a cryoprotective role in brine channels of sea ice, where extremes of high salinity and low temperature impose pressures on microbial growth and survival. The EPS produced at −2°C and 10°C had a higher uronic acid content than that produced at 20°C. The availability of iron as a trace metal is of critical importance in the Southern Ocean, where it is known to limit primary production. EPS from strain CAM025 is polyanionic and may bind dissolved cations such at trace metals, and therefore the presence of bacterial EPS in the Antarctic marine environment may have important ecological implications.
Sea ice is a major component of polar regions, covers millions of square kilometers even at the end of the polar summer (21), and provides a home to unique communities dominated by microorganisms (35). Sea ice begins to form as the temperature of seawater, with a salinity of 3.5%, drops to −1.8°C. As ice develops, a salting-out process occurs in which the marine salts excluded from the ice are concentrated in brine pockets. Brine channels develop as the ice rises above sea level. The dense brine drains through the layer of columnar congelation ice by gravity and flows to the underlying seawater (7). The sea ice produces highly variable microenvironments in terms of temperature, salinity, nutrient concentration, and light intensities within the columns, which may be as thick as 2 m. Salinity in sea ice brine can range from near that of freshwater to >15% at the ice-seawater interface (35). Temperatures can range from 0°C to −35°C. Sea ice is thus one of the coldest habitats on earth for marine life (22).
In spite of these extremes, up to 20 to 30% of primary production in sea ice cycles through heterotrophic bacteria (24, 30). Complex microbial communities, dominated by diatoms in close association with bacteria (30), are concentrated in the lower 10 to 20 cm of sea ice, where nutrients are available from the seawater and light is available from the surface (35). Abundant bacterial populations have been found in thick annual pack ice, with psychrophilic bacteria being particularly common in samples of pore waters and ice colored brown by the presence of algal pigments (15). During ice formation, microalgal cells are scavenged by sea ice crystals floating up to the sea surface (17), and bacteria attached to algal cells may be incorporated into new ice in conjunction with some algal species (18). Bacterially produced EPS may provide a means by which bacteria can adhere to the microalgal cells (36). Studies of both Arctic (26) and Antarctic (36) sea ice communities suggest that exopolymer production by both phytoplankton and bacteria makes a significant contribution to organic carbon in the sea ice and ice-water interfaces.
Little is known about EPS produced by Antarctic marine bacteria or about its role in this extreme environment. Sea ice bacteria maintained in laboratory culture can secrete copious amounts of mucus (20, 28). The sea ice isolate CAM025, examined in previous studies, belongs to the genus Pseudoalteromonas and class “Gammaproteobacteria” (28). Studies of polar sea ice communities using cultivation-dependent and -independent techniques have shown that the “Gammaproteobacteria” are among the dominant taxonomic groupings (6, 8, 10, 35). CAM025 exhibits growth in the temperature range of −2°C to 30°C, on media containing 1% to 12% (wt/vol) sea salts, and exhibits an enhanced mucoid morphology on marine media with added glucose (29).
When grown in batch culture at temperatures near the predicted optimum for this strain, CAM025 produced exopolymer, and chemical analyses showed the purified EPS was composed primarily of neutral sugars (glucose, arabinose, fucose, and galactose), uronic acids (glucuronic acid), and sulfates (29). Sulfates carry a net negative charge at seawater pH (27); uronic acids also contain an acidic carboxyl group that is ionizable in these conditions. The presence of these two groups (uronic acids and sulfates) is known to result in an EPS with a polyanionic quality (12). Due to these chemical characteristics, microbial exopolymer and EPS like those produced by CAM025 have been shown to accumulate cations such as metals (4, 9). The ecological role of EPS is therefore directly related to its chemistry. The frequency and type of functional groups present in the EPS impact on the tertiary structure and overall physicochemical characteristic of the polymer in the surrounding aqueous environment (12).
The EPS of strain CAM025 had a molecular mass of 5.7 × 106 daltons, which is high relative to that of EPS produced by many other marine bacteria (1 × 105 to 3 × 105 daltons) (12) but similar to those of EPS produced by bacteria from deep-sea hydrothermal vents (19). The physical, rheological, and chemical properties of EPS are influenced by the length of the polymer chain, that is, the molecular weight (11). As the length of the polymer increases, there is a greater opportunity for complex entanglement of polymer chains and intramolecular associations, and these contribute to the tertiary structure and physical behavior of the polymer (37). A fungal strain, Phoma herbarum, isolated from Antarctic soil produced a homosaccharide of glucose with a molecular mass of 7.4 × 106 daltons (33). The authors suggested the fungal EPS could provide a cryoprotective role in the harsh Antarctic environment, where liquid water is scarce and temperatures are extremely low.
Exopolymer produced by CAM025 in laboratory cultures incubated at 20°C has provided some insight into the possible ecological role for these biopolymers (29). Although 20°C is near the predicted optimum for growth of this psychrotolerant strain, it is much higher than that of the natural environment from which this strain was isolated. This study was undertaken to investigate the effects of a range of temperatures on the production of exopolymer and purified EPS by the sea ice isolate Pseudoalteromonas sp. strain CAM025.
MATERIALS AND METHODS
Batch cultures at different temperatures.
CAM025 was grown in duplicate broth cultures at −2°C, 10°C, and 20°C. Culture broth was composed of 1 g yeast extract (Oxoid L21); 5 g bacteriological peptone (Oxoid L37); 32 g artificial sea salts (Sigma S9883); 4.76 g HEPES (Sigma H7637); and 1,000 ml distilled water. The pH of the broth was adjusted to 8 prior to autoclaving. A glucose solution was prepared and autoclaved separately before being combined with the above media for a final concentration of 3% glucose (wt/vol). One-liter baffled flasks containing 220 ml of the above medium were inoculated with 20 ml of the exponentially growing batch cultures of the bacterial isolate. Approximately 5 ml of the broth culture was removed aseptically from each of the flasks to sterile McCartney bottles for pH, optical density (600 nm), viable cell, viscosity, and glucose measurements (described below) at inoculation and then once daily throughout the experiment. Flasks were incubated in oscillating water baths (Ratek Pty Ltd, Australia) fitted with refrigeration units that cooled and circulated antifreeze liquid at the desired temperature. All incubations took place in a room maintained at 20°C. Batch cultures at −2°C and 10°C were harvested after 2 weeks incubation. The cultures at 20°C were harvested after 1 week.
Enumeration.
The optical density of culture material was measured at 600 nm at 24-h intervals using a Smart Spec 3000 spectrophotometer (Bio-Rad, Regent Park, Australia). Aliquots (1 ml) of culture medium were removed from flasks at 48-h intervals, serially diluted in sterile artificial sea salts solution (3.2% in distilled water), and aliquots (150 μl) were spread onto replicate agar plates (marine agar with 3% [wt/vol] added glucose) with a Spiral Biotech automated plater. Cultures were incubated at 12°C for 4 days before colonies were enumerated for viable cell count determinations. The pH of culture material at 20°C was measured at 24 h intervals with an Orion pH electrode.
Chemical and physicochemical measurements.
The viscosity of a 1-ml aliquot of the culture broth, warmed to room temperature (20°C), was measured daily with a Brookfield LVT microviscometer fitted with a cone and plate assembly. (Middleboro, MA). One-ml aliquots of culture broth, removed every 24 h, were centrifuged at 15,000 × g for 10 min (Eppendorf, Hamburg, Germany). The supernatant was removed and frozen. Glucose was quantified using a Boehringer Mannheim kit (Darmstadt, Germany).
Isolation, purification, and characterization of CAM025 EPS.
Culture broth was centrifuged at 30,000 × g for 2 h at 4°C (Beckman Coulter). The cell pellets were freeze-dried and weighed. Culture broth was filtered and exopolymer was purified by filtration and dialysis and then freeze-dried and weighed. Uronic acid, protein, and total neutral carbohydrate content was determined and analysis of EPS monosaccharides was performed according to procedures described in detail (29).
Statistical treatment of data.
Univariate analysis of variance was performed on data gathered from measurements of cell yield, EPS yield, glucose consumption (initial glucose concentration minus final glucose concentration; [Glu]init -[Glu]final), crude chemical analysis, and monosaccharide analyses of EPS. The statistical analysis was carried out using the General Linear Model package of SPSS (34).
RESULTS
Growth of CAM025 in batch cultures.
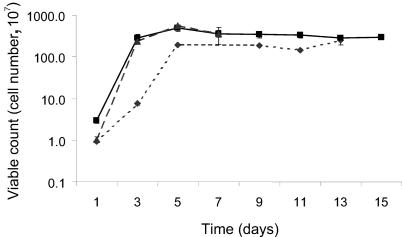
Viable cell counts showed that the highest rate of exponential growth occurred in cultures incubated at 10 and 20°C in the first 24 h (Fig. 1). Cultures at −2°C reached a maximum viable cell count by day 5 of incubation. The final optical density reading (600 nm) for each culture was used as an indication of the cell yield. The cell yield was significantly different between cultures incubated at −2°C and 10°C (Table 1).
FIG. 1.
Viable cell count for batch cultures of sea ice isolate CAM025, incubated at −2°C (⧫), 10°C (▪), and 20°C (▴).
TABLE 1.
Growth parameters of sea ice bacterium Pseudoalteromonas sp. strain CAM025 grown in duplicate batch cultures
| Parameter | Units | Value at incubation temp:
|
|||||
|---|---|---|---|---|---|---|---|
| −2°C
|
10°C
|
20°C
|
|||||
| Avg | SE | Avg | SE | Avg | SE | ||
| Cell yield | Absorbance (600 nm) | 4.68a | 0.3 | 10.13a | 0.4 | 7.7 | 1.0 |
| EPS yield | mg/g (dry weight) | 97.2b | 9.3 | 99.9c | 8.0 | 3.6b,c | 0.2 |
| Glucose consumption ([Glu]init − [Glu]final) | g/liter | 4.10d | 2.5 | 22.8d | 2.9 | 9.7 | 0.8 |
P ≤ 0.071.
P ≤ 0.024.
P ≤ 0.024.
P ≤ 0.076.
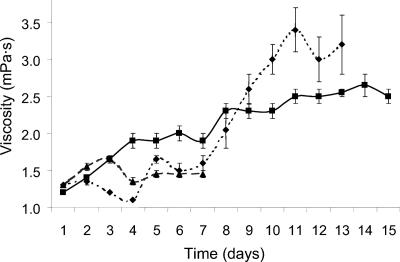
The viscosity of the culture medium from 20°C incubations reached a maximum of 1.7 millipascal-seconds (mPa.s) after 72 h (Fig. 2). A decrease on day 4 was followed by no change on the subsequent 3 days. Viscosity of cultures at 10°C increased gradually to a maximum of 2.7 mPa.s on day 14 and then decreased slightly. Cultures incubated at −2°C showed the greatest increase in viscosity, reaching a maximum of 3.4 mPa.s after 11 days.
FIG. 2.
Viscosity for batch cultures of sea ice isolate CAM025, incubated at −2°C (⧫), 10°C (▪), and 20°C (▴).
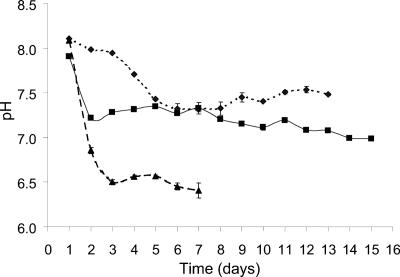
The pH of cultures at 10°C decreased 0.7 pH units within the first 24 h of incubation and then decreased gradually until day 15 (Fig. 3). The pH of cultures incubated at −2°C decreased gradually over the first 6 days and then gradually increased until day 13. The pH of 20°C cultures decreased 1.5 pH units by day 3 and after a slight increase on days 4 and 5, decreased over the subsequent 2 days. This decrease in pH was occurring without an increase in viscosity. The −2°C and 10°C cultures, in comparison, showed increases in viscosity and smaller decreases in pH (Fig. 3).
FIG. 3.
pH for batch cultures of sea ice isolate CAM025, incubated at −2°C (⧫), 10°C (▪), and 20°C (▴).
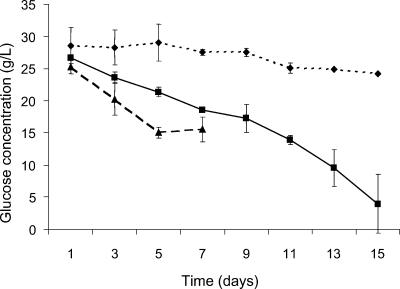
The concentration of glucose in the culture medium was measured throughout the the experiment (Fig. 4). The difference in glucose concentration in the batch cultures between the start and end of the incubation was calculated to represent the amount of glucose consumed at each temperature. Maximum glucose consumption occurred in cultures incubated at 10°C, where the final glucose concentration was 80% lower than at the start of the experiment. In contrast, a glucose consumption of 15% occurred in cultures incubated at −2°C. The difference in values representing glucose consumption at these two incubation temperatures was significant (Table 1).
FIG. 4.
Glucose concentration for batch cultures of sea ice isolate CAM025, incubated at −2°C (⧫), 10°C (▪), and 20°C (▴).
Yield and characterization of EPS.
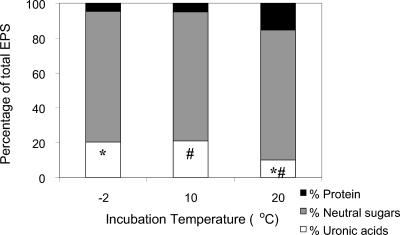
The yield of purified EPS at each temperature is expressed as mg of EPS per g (dry weight) of cell material. The yield of EPS at −2°C and 10°C was approximately 30-fold higher than at 20°C (Table 1). The crude chemical composition of EPS produced by replicate cultures incubated at three temperatures is shown as a percentage of the total (Fig. 5). Differences in total neutral sugar and protein content in the EPS produced by cultures at the three incubation temperatures were not significant. In contrast, uronic acid content in EPS expressed as percentage of total EPS produced at −2°C and 10°C was significantly different (P < 0.05) from and higher than at 20°C. Analysis of individual monosaccharides in EPS harvested from duplicate cultures incubated at −2°C, 10°C, and 20°C did not reveal any significant differences in the percentages of arabinose, ribose, and fucose. The percentages of mannose and glucose were higher in cultures incubated at −2°C than at 10°C or at 20°C, whereas the percentages of galactose and rhamnose were lower at −2°C than at 10°C or at 20°C. The percentages of galacturonic acid were lowest in cultures incubated at 20°C and highest in those incubated at −2°C (Table 2).
FIG. 5.
Crude chemical analysis of EPS produced by sea ice isolate CAM025 produced in batch cultures incubated at −2°C, 10°C, and 20°C, reported as percentage of total EPS. Pairs of symbols (* and #) denote significant (P < 0.05) differences in percentages.
TABLE 2.
Monosaccharide composition of EPS from duplicate cultures incubated at three temperaturesa
| Monosaccharide | % of total at incubation temp:
|
||
|---|---|---|---|
| −2°C | 10°C | 20°C | |
| Arabinose | 3.1 (1.0) | 3.7 (1.9) | 11.3 (2.5) |
| Ribose | 1.2 (0.9) | 1.6 (0.2) | 0.0 (0.0) |
| Rhamnose | 4.8 (0.5)b,c | 18.8 (3.1)b | 25.9 (5.8)c |
| Fucose | 1.6 (0.4) | 7.7 (0.4) | 6.9 (2.9) |
| Galacturonic acid | 15.8 (0.4)e,f | 8.1 (1.0)e,g | 0.2 (0.2)f,g |
| Mannose | 16.5 (0.7)h,j | 9.6 (1.5)h | 8.9 (0.8)j |
| Galactose | 5.7 (0.3)k,l | 19.7 (3.2)k | 23.6 (2.0)l |
| Glucose | 51.3 (1.8)m,n | 30.9 (5.4)m | 23.3 (9.2)n |
Data are % of total with standard errors of the means given in parentheses. Pairs of letters b to n denote significant differences (P < 0.05) between percentages of EPS monosaccharides from duplicate cultures.
DISCUSSION
Previous studies reported that the sea ice bacterium Pseudoaltermonas sp. strain CAM025 exhibited growth in the temperature range of −2 to 30°C, but no growth occurred on solid medium at 37°C (29). Other closely related Pseudoalteromonas species were shown to be psychrotolerant, that is, able to grow at 4°C and with a growth temperature optimum of approximately 22 to 25°C (5). Psychrotolerant bacteria appear to be common in both sea ice and the underlying water (10, 14, 20), with Pseudoalteromonas strains being the most frequently isolated within this group (6).
In the 10°C cultures, the exponential growth phase occurred in the first 24 h and was followed by a stationary phase in which viscosity increased. Cultures grown at 10°C also showed maximum optical density readings and highest cell yield, yield of EPS, and glucose utilization compared to the cultures at −2°C and 20°C (Table 1). A high yield of EPS accompanied by high cell yield appears to indicate balanced growth was occurring at this temperature compared to the −2°C and 20°C cultures. Maximum viscosity was reached during the stationary phase in all cultures, with cultures growing at −2°C and 10°C showing the highest viscosity.
In batch culture studies of the deep-sea hydrothermal vent isolate HYD 1545, EPS production began in the late exponential phase and continued during the stationary phase (38). The deep-sea hydrothermal vent strain Alteromonas sp. strain 1644 produced an EPS at the beginning of the stationary phase, and this suggested that synthesis was induced by restricted growth conditions (31). In the current study, cultures of CAM025 incubated at 20°C and pH 8.0 decreased by 1.7 units, while viscosity remained low during the early stationary phase. EPS production at 20°C seems to be restricted compared to production of EPS in cultures incubated at −2°C and 10°C.
In growth experiments with Lactobacillus sakei strain 0-1, low temperature combined with glucose as a carbohydrate source enhanced EPS production (13). In the observed growth temperature range of 15 to 42°C, 15 and 25°C were the best for bacterial growth and EPS production, respectively, for this mesophile. Specific production of EPS decreased with increasing temperature. Depletion of glucose indicated the C/N ratio of the medium was optimal (13). In the current study, glucose was depleted by 80% in cultures incubated at 10°C, and EPS and cell yield were the highest at this temperature. Cultures at −2°C showed the lowest glucose consumption and cell yield. At this temperature, CAM025 produced a high yield of EPS (Table 1). At −2°C, CAM025 may have had more energy to produce EPS as less was being used for production of cellular components than at higher incubation temperatures.
EPS production occurred during the stationary phase, as demonstrated by increasing viscosity after exponential growth had ceased. In addition, increased EPS yield at −2°C and 10°C indicated EPS production was occurring in these cultures at suboptimal temperatures. Optical density measurements for strain CAM025 indicated a higher cell yield for cultures incubated at 10°C compared to those incubated at 20°C. Previous studies of sea ice isolates of the genus Pseudoalteromonas and closely related to CAM025 suggested that the optimum temperature for growth would be in the 20°C to 22°C range (5). Further work is necessary before the precise optimal temperatures for cell growth and EPS production are known for strain CAM025.
In continuous fermentation studies with the mesophile Pseudomonas sp. strain NCIBI 11264, EPS production was influenced by medium composition, temperature, pH, and the growth rate of the organism. However, the polysaccharide varied little in overall composition irrespective of the pH, temperature, and nitrogen, carbon, and phosphate content of the growth medium (39). Culture conditions generally did not affect the types of monosaccharides in an EPS produced by the halophilic bacterium Halomonas maura (2). Crude chemical analysis of the EPS produced by CAM025 showed that there was a higher percentage of uronic acids in the EPS produced at −2°C and 10°C than at 20°C, while neutral sugar content did not vary significantly (Fig. 5). Further analyses of individual monosaccharides also showed the highest percentage of galacturonic acid in EPS produced by cultures incubated at −2°C relative to cultures incubated at 10°C or at 20°C. This coincided with a higher total neutral monosaccharide content at higher growth temperatures. Further study is required to more thoroughly assess the effect of growth temperature on individual monosaccharide contents.
During each austral summer, approximately four-fifths of the sea ice surrounding Antarctica melts (41), releasing dissolved and particulate material, living cells, and aggregations of cells into the surrounding waters (7). Arctic sea ice studies (25, 26) demonstrated that microbially produced neutrally buoyant EPS was carried large distances by prevailing under-ice currents and ice drifts. Studies in more temperate waters showed marine bacterial EPS production played a major role in aggregate formation (12, 3). When released into the water column, a combination of biological, chemical, and physical forces caused this colloidal material to form aggregates (1), which became centers of high heterotrophic microbiological activity (23).
The availability of iron as a trace metal is of critical importance in the Southern Ocean, where it is know to limit primary production (32). Since 99% of dissolved iron in the ocean is bound to organic ligands (40), polyanionic EPS such as those produced by CAM025 may have an important role in the Antarctic marine environment. Microbial EPS potentially act to keep iron in solution and accessible for primary productivity (16) or, in the framework of sinking aggregates, transporting bound iron out of the euphotic zone (40).
Conclusions.
CAM025 is a halotolerant, psychrotolerant sea ice isolate, belonging to the genus Pseudoalteromonas, which is known to abound in the sea ice microbial community. The enhanced production of a high-molecular-weight polyanionic EPS at suboptimal incubation temperatures lends support to theories that EPS may have a cryoprotective role in the high-salinity, low-temperature habitat of sea ice brine channels. The importance of the role of EPS in sea ice as well as in the Southern Ocean, where the availability of trace nutrients such as iron limits primary production and CO2 sequestration, requires further attention.
Acknowledgments
We thank Jenny Skerratt and Andrew Pankowski for sample collection. Sandrine Garon Lardière of IFREMER Centre de Brest is thanked for her help with carbohydrate analyses. Thushara Nair is thanked for her assistance with statistical analyses. June Olley and David Ratkowsky are thanked for helpful discussions in the preparation of the manuscript. Two anonymous reviewers are thanked for comments that improved the manuscript.
C.M.N. was supported by a Tasmanian Postgraduate Research Scholarship and by funding provided by the Australian Antarctic Division. C.M.N. also received a travel award from the Australian Academy of Science and the French Embassy in Canberra, Australia.
REFERENCES
- 1.Alldredge, A., and G. A. Jackson. 1995. Aggregation in marine systems. Deep-Sea Res. Pt. II 42:1-7. [Google Scholar]
- 2.Arias, S., A. del Moral, M. R. Ferrer, R. Tallon, E. Quesada, and V. Béjar. 2003. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 7:319-326. [DOI] [PubMed] [Google Scholar]
- 3.Biddanda, B. A. 1986. Structure and function of microbial aggregates. Oceanol. Acta 9:209-211. [Google Scholar]
- 4.Bitton, G., and V. Friehofer. 1978. Influence of extracellular polysaccharide on the toxicity of copper and cadmium toward Klebsiella aerogenes. Microb. Ecol. 4:119-125. [DOI] [PubMed] [Google Scholar]
- 5.Bowman, J. P. 1998. Pseudoalteromonas prydzensis sp. nov., a psychrotrophic, halotolerant bacterium from Antarctic sea ice. Int. J. Syst. Bacteriol. 48:1037-1041. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, J. P., S. A. McCammon, M. V. Brown, D. S. Nichols, and T. A. McMeekin. 1997. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 63:3068-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brierley, A. S., and D. N. Thomas. 2002. Ecology of Southern Ocean pack ice. Adv. Mar. Biol. 43:171-280. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmeyer, R., K. Knittel, J. Jurgens, H. Weyland, R. Amann, and E. Helmke. 2003. Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl. Environ. Microbiol. 69:6610-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, M. J., and J. N. Lester. 1982. Role of bacterial extracellular polymers in metal uptake in pure bacterial culture and activated sludge-I. Water Res. 16:1539-1548. [Google Scholar]
- 10.Brown, M. V., and J. P. Bowman. 2001. A molecular phylogenetic survey of sea ice microbial communities (SIMCO). FEMS Microbiol. Ecol. 35:267-275. [DOI] [PubMed] [Google Scholar]
- 11.Christensen, B. E. 1999. Physical and chemical properties of extracellular polysaccharides associated with biofilms and related substances, p. 144-154. In J. Wingender, T. Neu, and H.-C. Flemming (ed.), Microbial extracellular substances: characterization, structure and function. Springer, New York, NY.
- 12.Decho, A. W. 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes, p. 73-153. In M. Barnes (ed.), Oceanography and marine biology annual review. Aberdeen University Press, Aberdeen, Scotland.
- 13.Degeest, B., B. Janssens, and L. De Vuyst. 2001. Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0-1: production kinetics, enzyme activities, and EPS yields. J. Appl. Microbiol. 91:470-477. [DOI] [PubMed] [Google Scholar]
- 14.Delille, D. 1996. Biodiversity and function of bacteria in the Southern Ocean. Biodivers. Conserv. 5:1505-1523. [Google Scholar]
- 15.Delille, D. 1992. Marine bacterioplankton at the Weddell sea ice edge, distribution of psychrophilic and psychrotrophic populations. Polar Biol. 12:205-210. [Google Scholar]
- 16.Geider, R. J. 1999. Complex lessons of iron uptake. Nature 400:815-816. [Google Scholar]
- 17.Gleitz, M., and D. N. Thomas. 1993. Variation in phytoplankton standing stock, chemical composition and physiology during sea-ice formation in the southeastern Weddell Sea. J. Exp. Mar. Biol. Ecol. 173:211-230. [Google Scholar]
- 18.Grossmann, S., and G. S. Dieckmann. 1994. Bacterial standing stock, activity and carbon production during formation and growth of sea ice in the Weddell Sea. Appl Environ Microbiol. 60:2746-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guezennec, J. 2002. Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest? J. Ind. Microbiol. Biol. 29:204-208. [DOI] [PubMed] [Google Scholar]
- 20.Helmke, E., and H. Weyland. 1995. Bacteria in the sea ice and underlying water on the eastern Weddell Sea in midwinter. Mar. Ecol. Prog. Ser. 117:269-287. [Google Scholar]
- 21.Horner, R. A., S. F. Ackley, G. S. Dieckmann, B. Gulliksen, T. Hoshaii, L. Legendre, I. A. Melnikov, W. S. Reeburgh, M. Spindler, and C. W. Sullivan. 1992. Ecology of sea ice biota. 1. Habitat, terminology and methodology. Polar Biol. 12:417-427. [Google Scholar]
- 22.Junge, K., J. F. Imhoff, J. T. Staley, and J. W. Deming. 2002. Phylogenetic diversity of numerically important bacteria in Arctic sea ice. Microb. Ecol. 43:315-328. [DOI] [PubMed] [Google Scholar]
- 23.Kiorboe, T. 2001. Formation and fate of marine snow: small-scale processes with large scale implications. Sci. Mar. 65:57-71. [Google Scholar]
- 24.Kottmeier, S. T., S. M. Grossi, and C. W. Sullivan. 1987. Sea ice microbial communities. VIII. Bacterial production in annual sea ice of McMurdo Sound, Antarctica. Mar. Ecol. Prog. Ser. 36:287-298. [Google Scholar]
- 25.Krembs, C., H. Eicken, K. Junge, and J. W. Deming. 2002. High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep-Sea Res. Pt. I 49:2163-2181. [Google Scholar]
- 26.Krembs, C., and A. Engel. 2001. Abundance and variability of microorganisms and transparent exopolymer particles across ice-water interface of melting first-year sea ice in the Laptev Sea (Arctic). Mar. Biol. 138:173-185. [Google Scholar]
- 27.Leppard, G. G., A. Heissenberger, and G. J. Herndl. 1996. Ultrastructure of marine snow. I. Transmission electron microscopy methodology. Mar. Ecol. Prog. Ser. 135:289-298. [Google Scholar]
- 28.Mancuso Nichols, C., S. Garon Lardiere, J. P. Bowman, P. D. Nichols, J. A. E. Gibson, and J. Guézennec. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb. Ecol., in press. [DOI] [PubMed]
- 29.Mancuso Nichols, C., S. Garon, J. P. Bowman, G. Raguénès, and J. Guézennec. 2004. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 96:1057-1066. [DOI] [PubMed] [Google Scholar]
- 30.Palmisano, A. C., and D. L. Garrison. 1993. Microorganisms in Antarctic sea ice, p. 167-219. In E. Friedmann (ed.), Antarctic microbiology. Wiley-Liss, New York, NY.
- 31.Samain, E., M. Milas, L. Bozzi, M. Dubreucq, and M. Rinaudo. 1997. Simultaneous production of two different gel-forming exopolysaccharides by an Alteromonas strain originating from deep-sea hydrothermal vents. Carbohydr. Polymers 34:235-241. [Google Scholar]
- 32.Scharek, R., M. A. Vanleeuwe, and H. J. W. Debaar. 1997. Responses of southern ocean phytoplankton to the addition of trace metals. Deep-Sea Res. Pt. II 44:209-227. [Google Scholar]
- 33.Selbmann, L., S. Onofri, M. Fenice, F. Frederico, and M. Petriuccioli. 2002. Production and structural characterization of the exopolysaccharde of the Antarctic fungus Phoma herbarum CCFEE 5080. Res. Microbiol. 153:585-592. [DOI] [PubMed] [Google Scholar]
- 34.SPSS. 2004. Handbook. SPSS Inc., Chicago, Illinois.
- 35.Staley, J. T., and J. J. Gosink. 1999. Poles apart: Biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53:189-215. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan, C. W., and A. C. Palmisano. 1984. Sea ice microbial communities: distribution, abundance and diversity of ice bacteria in McMurdo Sound, Antarctica, in 1980. Appl. Environ. Microbiol. 47:788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland, I. W. 1994. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv. 12:393-448. [DOI] [PubMed] [Google Scholar]
- 38.Vincent, P., P. Pignet, F. Talmont, L. Bozzi, B. Fournet, and J. G. Guezennec. 1994. Production and characterization of an exopolysaccharide excreted by a deep-sea hydrothermal vent bacterium isolated from the polychaete annelid Alvinella pompejana. Appl. Environ. Microbiol. 60:4134-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, A. G., and J. T. W. Wimpenny. 1978. Exopolysaccharide production by Pseudomonas NCIB11264 grown in continuous culture. J. Gen. Microbiol. 104:47-57. [DOI] [PubMed] [Google Scholar]
- 40.Wu, J. F., E. Boyle, W. Sunda, and L. S. Wen. 2001. Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science 293:847-849. [DOI] [PubMed] [Google Scholar]
- 41.Zwally, H. J., J. C. Comiso, C. L. Parkinson, W. J. Cambell, F. D. Carsey, and P. Gloersen. 1983. Antarctic sea-ice, 1973-1976: Satellite passive-microwave observations. Report SP-459. National Aeronautics and Space Administration Scientific and Technical Information Branch. National Aeronautics and Space Administration, Washington, D.C.