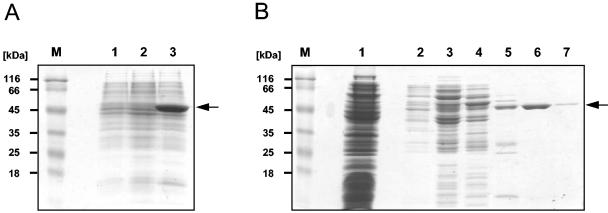
FIG. 2.
Overproduction and purification of M. pneumoniae GlpK. (A) Sodium dodecyl sulfate (SDS)- polyacrylamide gel electrophoresis for the detection of His6-tagged GlpK in crude extracts of E. coli DH5α bearing either the empty expression vector pWH844 (lane 1); the expression vector including the wild-type glpK allele, pGP253 (lane 2); or the vector including the mutated glpK allele, pGP254 (lane 3). Cells were grown to an optical density at 600 nm of 0.8, and expression from the IPTG-inducible promoter was induced by addition of 1 mM IPTG (final concentration). After 2 h, cells were harvested and disrupted by sonication. The insoluble fraction was pelleted in a centrifugation step and solubilized using 6 M urea, and sample aliquots were separated on an SDS-12% polyacrylamide gel. (B) SDS-polyacrylamide gel electrophoresis to monitor the purification of His6-tagged GlpK. Crude extract of the GlpK expression strain (E. coli DH5α bearing the plasmid pGP254) that had been grown in the presence of 1 mM IPTG was passed over a Ni2+-nitrilotriacetic acid superflow column (5-ml bed volume; QIAGEN) and washed extensively with a buffer containing 10 mM Tris-HCl (pH 7.4) and 200 mM NaCl, followed by elution with an imidazole gradient (from 10 to 500 mM imidazole). Aliquots of the individual fractions were separated on SDS-12% polyacrylamide gels. A prestained protein molecular mass marker (Fermentas) served as a standard (lane M). Lane 1, flowthrough; lane 2, 10 mM imidazole; lane 3, 20 mM imidazole; lane 4, 50 mM imidazole; lane 5, 100 mM imidazole; lane 6, 200 mM imidazole; lane 7, 500 mM imidazole.