Abstract
We describe a renal transplant patient with a primary Toxoplasma gondii infection presenting as pneumonitis, with subsequent chorioretinitis and encephalitis. The diagnostic challenges of T. gondii infection in immunocompromised patients are discussed.
CASE REPORT
A 58-year-old female with end stage renal failure due to polycystic kidney disease received a renal transplant from a living donor. No acute rejection episodes occurred. Her immunosuppressive medication consisted of methylprednisolone, tacrolimus, and mycophenolate mofetil. Trimethoprim-sulfamethoxazole (TMP-SMZ) at 480 mg daily was continued for 3 months after transplantation as Pneumocystis carinii pneumonia prophylaxis. Eight days after the operation, the patient left the hospital in good clinical condition. Prednisone was tapered off to 5 mg twice a day and tacrolimus to trough levels between 5 and 10 ng/ml. Two months after transplantation, her condition was so good that she successfully participated in a 4-day walking tour of 120 kilometers. Four months after transplantation, she reported influenza-like symptoms with night sweats, headache, and a nonproductive cough. New-onset diabetes was diagnosed, for which gliclazide was started and tacrolimus was converted to cyclosporine. Shortly afterward, she was admitted to the hospital with a fever of 40°C, moderate weight loss, and dyspnea. Physical examination showed no further abnormalities. Laboratory examination revealed an elevated lactate dehydrogenase activity of 862 U/liter (normal value, <450 U/liter) and a C-reactive protein level of 74 g/liter with normal thrombocyte and leukocyte counts. During admission, her clinical condition deteriorated with hypotension, dyspnea, and progressive bilateral interstitial infiltrates on X-ray. The C-reactive protein level increased to a maximum of 213 g/liter with severe thrombocytopenia (21 × 109) and a lactate dehydrogenase activity of 3,909 U/liter with a normal haptoglobin concentration (2.2 g/liter). She received empirical treatment with an antibiotic regimen including amoxicillin, ceftazidime, ciprofloxacin, and high-dose TMP-SMZ. Because of respiratory failure, she was temporarily treated with noninvasive mechanical ventilation. The cause of the interstitial pneumonia remained unexplained. Repeated cultures of bronchoalveolar lavage (BAL) fluid for fungi, bacteria, and viruses and stains for P. carinii were negative, while serologic evaluation for cytomegalovirus and respiratory pathogens was inconclusive.
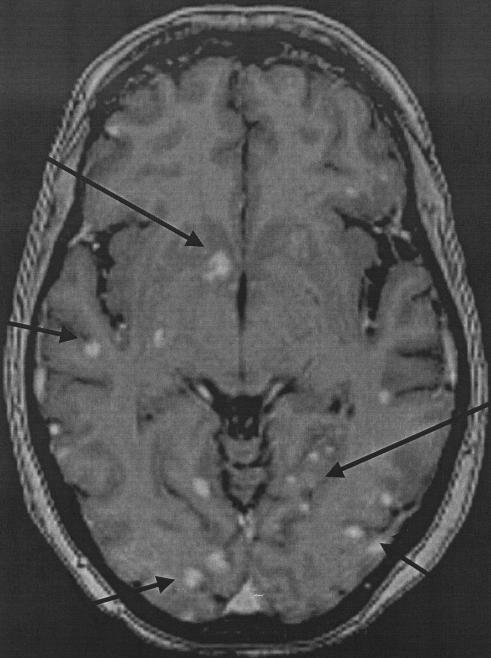
She slowly recovered and was then transferred to a rehabilitation center because of a presumed critical illness neuropathy. One month later, she was readmitted with syncope, headache, generalized weakness, slurred speech, and a fever of 39°C. On neurological examination, she showed a diffuse encephalopathy with altered consciousness and slight dysarthria but no focal neurological signs. A subsequent computed tomography scan showed multiple small hypodense lesions in the basal ganglia and one larger lesion in the cerebellum. A magnetic resonance image (MRI) of the brain showed numerous miliary lesions with a hyperintense signal on T1-weighted images in basal ganglia, the cerebello-occipital area, and more diffusely in the cortical and subcortical areas (Fig. 1). The cerebrospinal fluid (CSF) showed an elevated protein level (752 mg/liter) with elevated biochemical markers of neuronal and glial damage but no leukocytosis and a normal glucose level. Funduscopy showed chorioretinitis.
FIG. 1.
MRI of the cerebrum with multiple smaller and larger miliary lesions (arrows).
Empirical therapy with ceftriaxone, voriconazole, and TMP-SMZ was started. Cyclosporine was stopped, and prednisone was increased to 10 mg twice a day. A surgical biopsy of a cerebellar lesion showed necrosis with nonspecific inflammation and no sign of malignant disease. Diagnostic procedures, as summarized in Table 1, were negative. At this point, toxoplasmosis was considered a diagnostic possibility. Serologic testing for Toxoplasma gondii antibodies showed a low immunoglobulin G (IgG) level and no IgM, radiological imaging was not deemed typical, and PCR assays for T. gondii were negative with both CSF and a brain biopsy sample. At this point, it was not known whether the renal transplant donor or the recipient was seronegative or seropositive for T. gondii at the time of transplantation. Further tests were done to determine if both the episode of unexplained respiratory illness and the current neurological disease and chorioretinitis fitted toxoplasmosis.
TABLE 1.
Differential diagnosis of infectious causes of the cerebral lesions and performed tests
| Diagnosis | Performed tests |
|---|---|
| Bacterial | |
| Multiple pyogenic brain abscesses (septic emboli, nocardiae, | CSF and blood “cultures Gram staining of brain biopsy sample |
| actinomycosis) | |
| Neurosyphilis | Serology (TPPA)a |
| Mycobacterial infections | CSF culture, Ziehl-Nielsen staining of brain biopsy sample |
| Borrelia burgdorferi | Serology |
| Fungal | CSF and brain biopsy cultures, Grocott staining of brain biopsy sample |
| Aspergillosis | Galactomannan test of CSF and blood samples |
| Cryptococcosis | Antigen test of blood and CSF samples |
| Viral | |
| EBVb-related lymphoma | PCR assay of CSF and plasma samples |
| Varicella-zoster virus-related vasculitis | PCR, serology |
| HIVc | Serology |
| Parasitic | |
| Toxoplasmosis | PCR assay of CSF sample, PASd staining of brain biopsy sample |
TPPA, Treponema pallidum particle agglutination assay.
EBV, Epstein-Barr virus.
HIV, human immunodeficiency virus.
PAS, periodic acid-Schiff.
In our department, all serum samples are stored for at least 3 years and BAL fluid is frozen and stored for 3 months. Therefore we could test several serum samples from the pa-tient, including samples taken before transplantation from the patient and the donor. Antibodies for T. gondii were measured with a Vidas assay (bio-Merieux, Marcy-l'Etoile, France) according to the manufacturer's instructions with a toxoplasma serology kit for IgG and IgM and IgG avidity in subsequent serum samples. PCR for the T. gondii B1 gene was performed as previously described (11) with stored plasma, BAL fluid, CSF, and brain biopsy samples. The recipient was found to be seronegative at the time of transplantation, whereas the donor was positive for toxoplasma IgG both in a sample taken 2 years before transplantation and on the day of transplantation. Results are shown in Tables 2 and 3.
TABLE 2.
Serologic testing for T. gondii in subsequent samplesa
| Day of illnessb | IgG level (IU/ml) | IgM detection | Avidityd |
|---|---|---|---|
| 0 | Neg | Neg | NDc |
| 9 | 7 | Pos | ND |
| 16 | 28 | Pos | 0.112 |
| 41 | 68 | Neg | 0.033 |
| 56 | 81 | Neg | ND |
| 71 | 96 | ND | 0.008 |
| 106 | 95 | ND | 0.015 |
| 127 | 80 | ND | 0.013 |
| 148 | 85 | ND | 0.005 |
| 255 | 54 | ND | 0.022 |
| 290 | 51 | ND | 0.033 |
| 329 | 47 | ND | 0.067 |
At day 1, the patient was admitted with pneumonia; at day 53, she was readmitted with neurological symptoms and chorioretinitis. Neg, negative; Pos, positive.
Day of onset of illness was day 133 posttransplantation.
ND, not done.
Values of <0.200 are low avidity.
TABLE 3.
PCR testing for T. gondii in subsequent samples
| Day of illnessa | Tested sample(s) | Result of PCR assay for T. gondiib |
|---|---|---|
| 1 | Plasma | Neg |
| 6 | BAL fluid | Pos |
| 8 | Plasma | Pos |
| 11 | BAL fluid | Pos |
| 16 | Plasma | Pos |
| 54 | CSF, brain biopsy | Neg |
Day of onset of illness was day 133 posttransplantation.
Neg, negative; Pos, positive.
A diagnosis of primary pulmonary toxoplasmosis with parasitemia, subsequent cerebral toxoplasmosis, and chorioretinitis was made based on seroconversion complemented by a positive PCR assay of BAL fluid and plasma samples. Avidity was low (<0.200) in all tested serum samples of the transplant recipient, including a sample taken 10 months after infection.
The patient was treated successfully with pyrimethamine at 75 mg/day and sulfadiazine at 1 g four times a day for 3 months. She made a full neurological recovery, but she lost part of her vision in both eyes due to the chorioretinitis.
T. gondii is a well-known opportunistic pathogen in AIDS and heart transplant patients, but it remains a rare but significant pathogen in renal transplant recipients. Surveillance studies of renal transplant recipients in other countries show asymptomatic infection in 2 to 8% (3, 10). In one series, 10 to 14% of seropositive patients showed serologic evidence of reactivation (rise in IgG titers) but only three patients (1.5%) developed clinical disease (10). So far, only 35 cases of visceral and cerebral toxoplasmosis complicating renal transplantation have been described.
Lack of clinical awareness and difficulties in establishing a diagnosis are thought to contribute to the high mortality of up to 65%. This is illustrated by the fact that 15 of 35 cases (43%) were diagnosed at autopsy (1, 2, 5, 10).
Toxoplasmosis in transplant patients may result from reactivation of latent infection or from primary infection. So far, in renal transplant recipients 7 cases (20%) have occurred in seropositive recipients and 16 (46%) in seronegative recipients while in 34% the serology was unknown (1, 2, 5, 10). Out of 16 cases with a primary infection, 15 had a seropositive donor and these infections were thought to be transplant related. Primary T. gondii infection has been documented between 1 day and 13 months after transplantation (median, 40 days). Reactivations have been described up to 7 years after transplantation (1, 2, 5, 10). In The Netherlands, renal transplant donors and recipients are not screened for toxoplasmosis since T. gondii infections in renal transplant recipients are thought to be rare. Because serum samples of our patient were stored, it could be determined that she was seronegative before transplantation and had a seropositive donor and it could be concluded that she suffered from a primary infection with the transplant as the most likely source of infection. The (long) interval of 4 months between transplantation and disease may be explained by the fact that the patient used P. carinii pneumonia prophylaxis for 3 months. TMP-SMZ given at a higher dosage and over a longer period of time has been reported to be effective in prevention of toxoplasmosis in cardiac transplant recipients (6, 9).
The clinical presentation of toxoplasmosis in renal transplant recipients varies. Fever is the most frequent clinical sign (80%), followed by pneumonia and generalized neurological signs such as headaches, drowsiness, and lethargy. Radiographic images of T. gondii pneumonia usually demonstrate interstitial infiltrates, but other patterns have been described (7, 8). In cerebral infection, typically deep-seated lesions with an asymmetric target sign, with variable signal intensity of T2-weighted images in the MRI are seen. Atypical manifestations with multiple miliary lesions have been described in bone marrow transplant patients and AIDS patients but so far not in renal transplant recipients (4, 14).
The diagnosis of T. gondii infection can be established by serology, by direct visualization of the parasite in tissue or clinical specimens, or by specific nucleic acid amplification by PCR assay for T. gondii. In the case of reactivation, the value of serology is limited (7, 8) but it can be used to diagnose a primary infection by showing seroconversion. In immunocompromised patients, absence of specific antibodies does not exclude active disease. In our patient, it took 8 days from the appearance of initial symptoms of fever and pneumonia for an antibody response to develop. At the time of cerebral toxoplasmosis, IgM was no longer present. IgG antibodies had a low avidity, which is thought to be indicative of recent infection. It is of note, however, that IgG was still of low avidity after 10 months, which indicates that low-to-high avidity switching in immunocompromised patients might be delayed (normally, 4 to 6 months) (8), making interpretation of serology results even more difficult. When serology is inconclusive, additional tests are needed. In our case, direct examination of BAL fluid for parasites with a Giemsa stain was negative but PCR assays of archival BAL fluid and plasma samples were positive (Table 3). It is not clear how long T. gondii can be detected in blood. In studies with rhesus monkeys, parasitemia was found for 14 days after primary infection (12). So far, four other renal transplant recipients have been reported with positive PCR assays of blood, two cases with a reactivation and two with a primary infection. The positive samples were collected 10 to 30 days after onset of disease (1). PCR assay of blood could therefore be a valuable diagnostic tool in the acute phase of primary infection.
Despite the extensive cerebral lesions in our patient, PCR assays of brain biopsy and CSF samples were negative. Sampling error may have been the cause. We have previously shown by PCR that T. gondii is only intermittently present in the CSF (13) and that testing of repeated samples can elevate the assay's sensitivity.
Toxoplasmosis should be considered in the differential diagnosis of pneumonia, culture-negative sepsis, and encephalitis in renal transplant recipients. Disseminated cerebral infections may present with a radiological image of small miliary lesions, much different from the “classical” deep-seated ring-enhanced lesion with a asymmetric target sign. PCR assay of CSF is not always positive, but PCR assays of BAL fluid and plasma can be a valuable aid in making an early diagnosis. When T. gondii IgG antibodies are present, archival serum samples can help to make a diagnosis of primary toxoplasmosis by demonstrating seroconversion but antibodies can be absent early in the infection and the interpretation of IgG avidity is not as straightforward as suggested in the literature. In The Netherlands, pretransplant screening for T. gondii is deemed not to be cost effective because of the low seroprevalence. However, screening will help in identifying patients at risk, especially seronegative recipients with seropositive donors, and can help in establishing the diagnosis by showing seroconversion. If no standard screening is performed, specimens should be stored for later testing. Increased awareness and early diagnosis may improve an otherwise poor outcome of toxoplasmosis in renal transplant recipients.
REFERENCES
- 1.Aubert, D., F. Foudrinier, I. Villena, J. M. Pinon, M. F. Biava, and E. Renoult. 1996. PCR for diagnosis and follow-up of two cases of disseminated toxoplasmosis after kidney grafting. J. Clin. Microbiol. 34:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Da Cunha, S., E. Ferreira, I. Ramos, R. Martins, L. de Freitas, J. Luis Borges, R. Corte-Real, A. Mota, A. Melico-Silvestre, and A. Linhares Furtado. 1994. Cerebral toxoplasmosis after renal transplantation. Acta Med. Port. 7:61-66. [PubMed] [Google Scholar]
- 3.Derouin, F., A. Debure, E. Godeaut, M. Larivire, and H. Kreis. 1987. Toxoplasma antibody titers in renal transplant recipients. Pretransplant evaluation and post-transplant follow-up of 73 patients. Transplantation 44:515-518. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich, U., M. Maschke, A. Dorfler, M. Prumbaum, and M. Forsting. 2002. MRI of intracranial toxoplasmosis after bone marrow transplantation. Neuroradiology 42:14-18. [DOI] [PubMed] [Google Scholar]
- 5.Giordano, L. F. C. M., E. P. Lasmar, E. R. F. Tavora, and M. F. Lasmar. 2002. Toxoplasmosis transmitted via kidney allograft: case report and review. Transplant. Proc. 34:498-499. [DOI] [PubMed] [Google Scholar]
- 6.Keogh, A., P. Macdonald, D. Richens, A. Harvison, and P. Spratt. 1992. Mini-dose trimethoprim with sulfamethoxazole prevents pneumocystis and toxoplasmosis after heart transplantation. Transplant. Proc. 24:2263. [PubMed] [Google Scholar]
- 7.Lumbreras, C., and J. M. Aguado. 2003. Toxoplasmosis after solid organ transplantation, p. 541-552. In R. A. Bowden, P. Ljungman, and C. V. Paya (ed.), Transplant infections, 2nd edition. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 8.Montoya, J. G., and J. S. Remington. 2000. Toxoplasma gondii, p. 2858-2881. In G. L. Mandell, D. L. Gordon, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th edition, vol 2. Churchill Livingstone, Philadelphia, Pa.
- 9.Munoz, P., J. Arencibia, C. Rodriguez, M. Rivera, J. Palomo, J. Yanez, and E. Bouza. 2003. Trimethoprim-sulfamethoxazole as toxoplasmosis prophylaxis for heart transplant recipients. Clin. Infect. Dis. 36:932-933. [DOI] [PubMed] [Google Scholar]
- 10.Renoult, E., E. Georges, M.-F. Biava, C. Hulin, L. Frimat, D. Hestin, and M. Kessler. 1997. Toxoplasmosis in kidney transplant recipients: report of six cases and review. Clin. Infect. Dis. 24:625-634. [DOI] [PubMed] [Google Scholar]
- 11.Schoondermark-van de Ven, E. M. E., J. Galama, W. Camps, J. Meuwissen, and W. Melchers. 1993. Diagnosis of Toxoplasma gondii infection by molecular detection, p. 199-207. In J. E. Smith (ed.), Toxoplasmosis: recent advances. Proceedings of the NATO-ARW on Toxoplasmosis. Springer-Verlag, London, United Kingdom.
- 12.Schoondermark-van de Ven, E. M., W. J. Melchers, J. M. Galama, J. H. Meuwissen, and T. K. Eskes. 1997. Prenatal diagnosis and treatment of congenital Toxoplasma gondii infections: an experimental study in rhesus monkeys. Eur. J. Obstet. Gynecol. Reprod. Biol. 74:183-188. [DOI] [PubMed] [Google Scholar]
- 13.Schoondermark-van de Ven, E., J. Galama, C. Kraaijeveld, J. Meuwissen, and W. Melchers. 1993. Diagnostic value of the polymerase chain reaction for the detection of Toxoplasma gondii in cerebrospinal fluid of patients with AIDS. Clin. Infect. Dis. 16:661-666. [DOI] [PubMed] [Google Scholar]
- 14.Seong, D. C., D. Przepiorka, J. M. Bruner, P. Van Tassel, W. K. Lo, and R. M. Champlin. 1993. Leptomenigeal toxoplasmosis after allogeneic bone marrow transplantation. Am. J. Clin. Oncol. 16:105-108. [DOI] [PubMed] [Google Scholar]