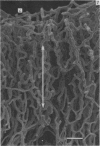
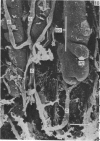
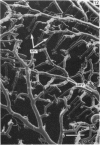
Abstract
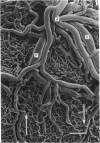
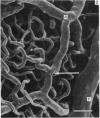
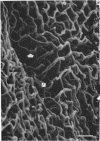
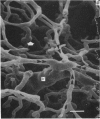
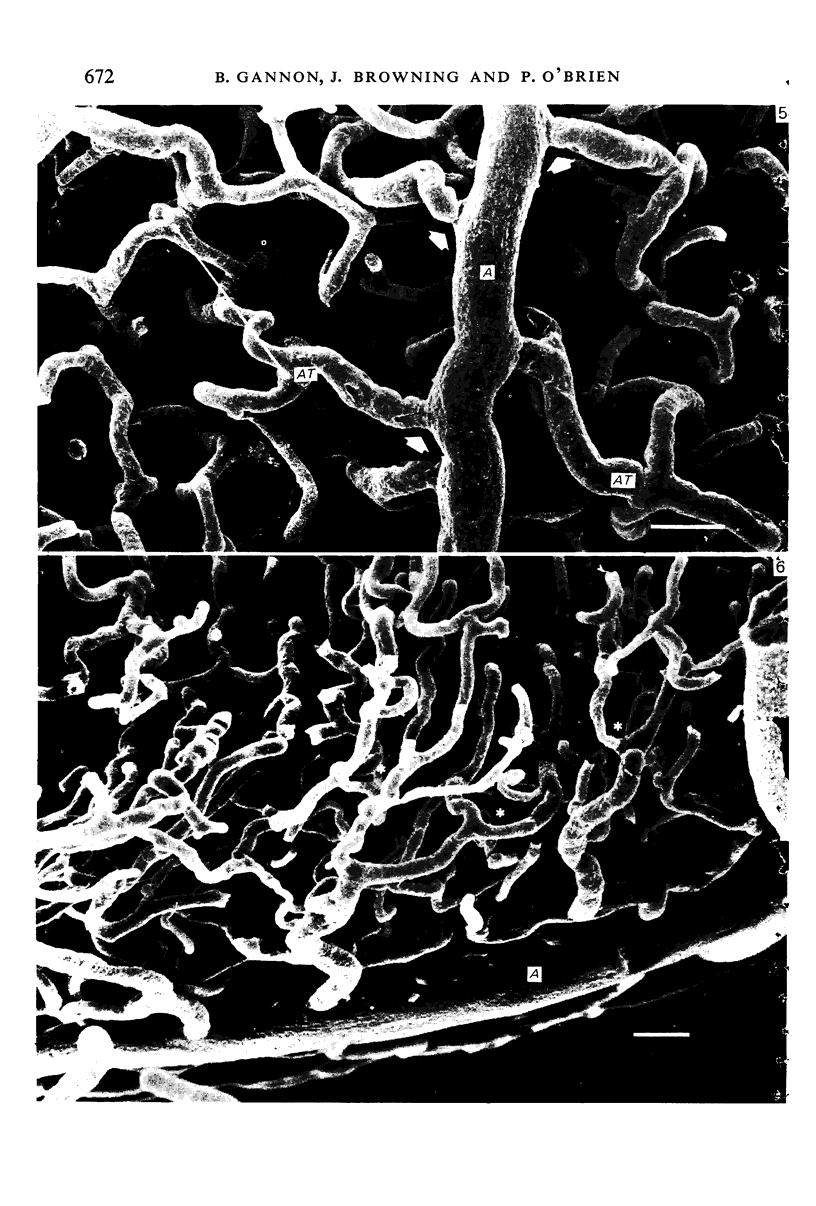
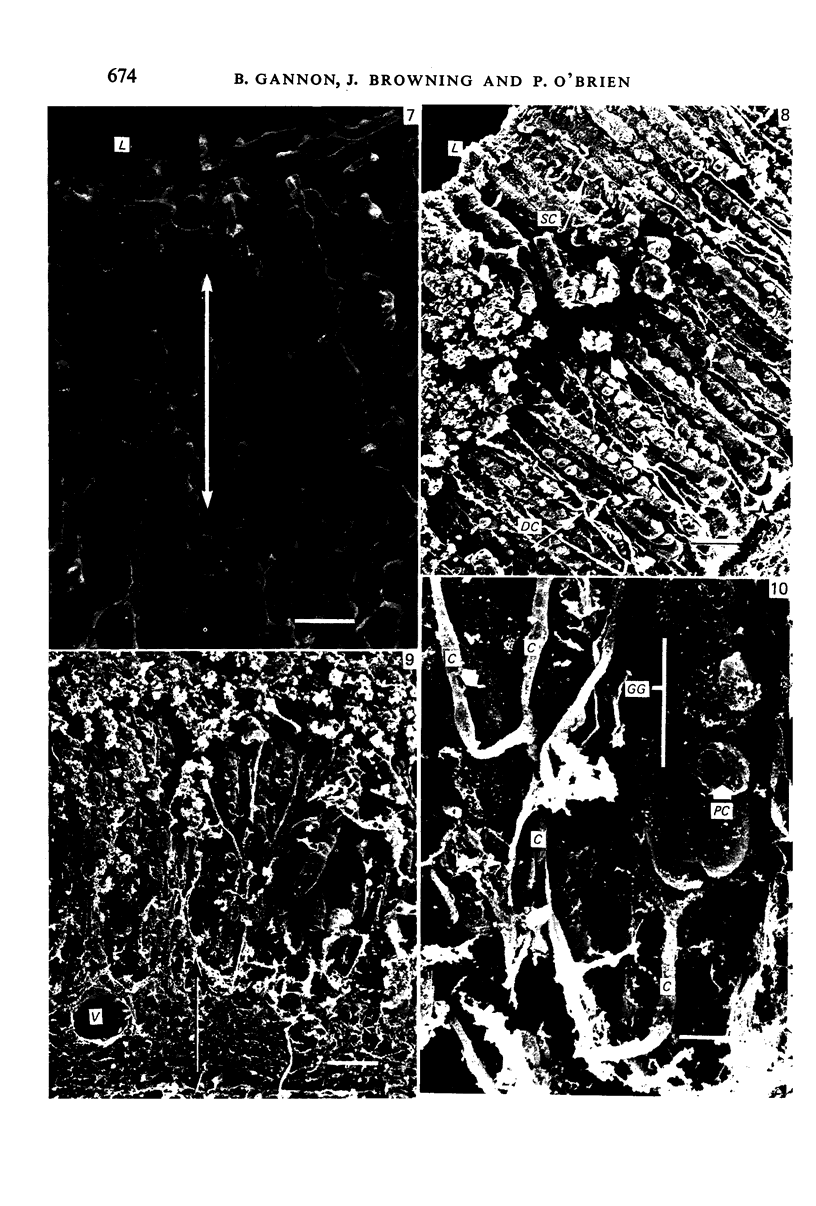
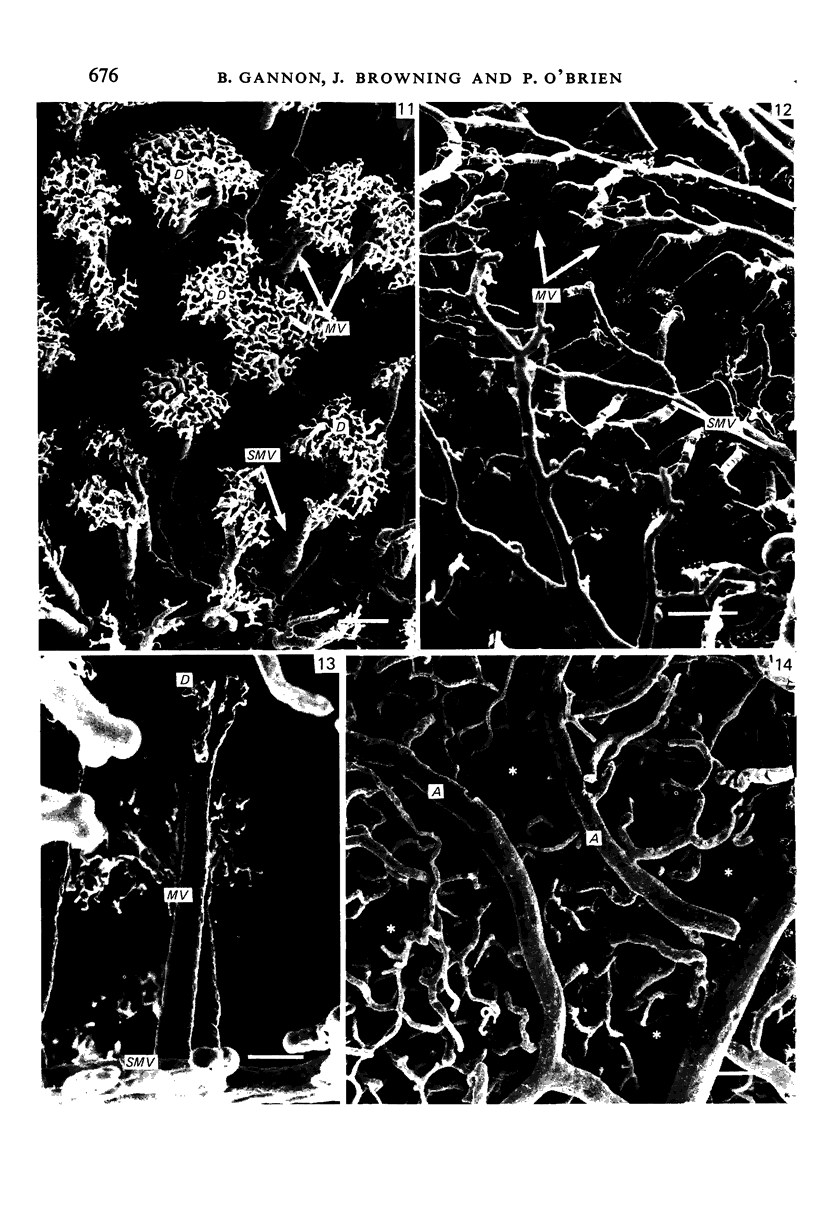
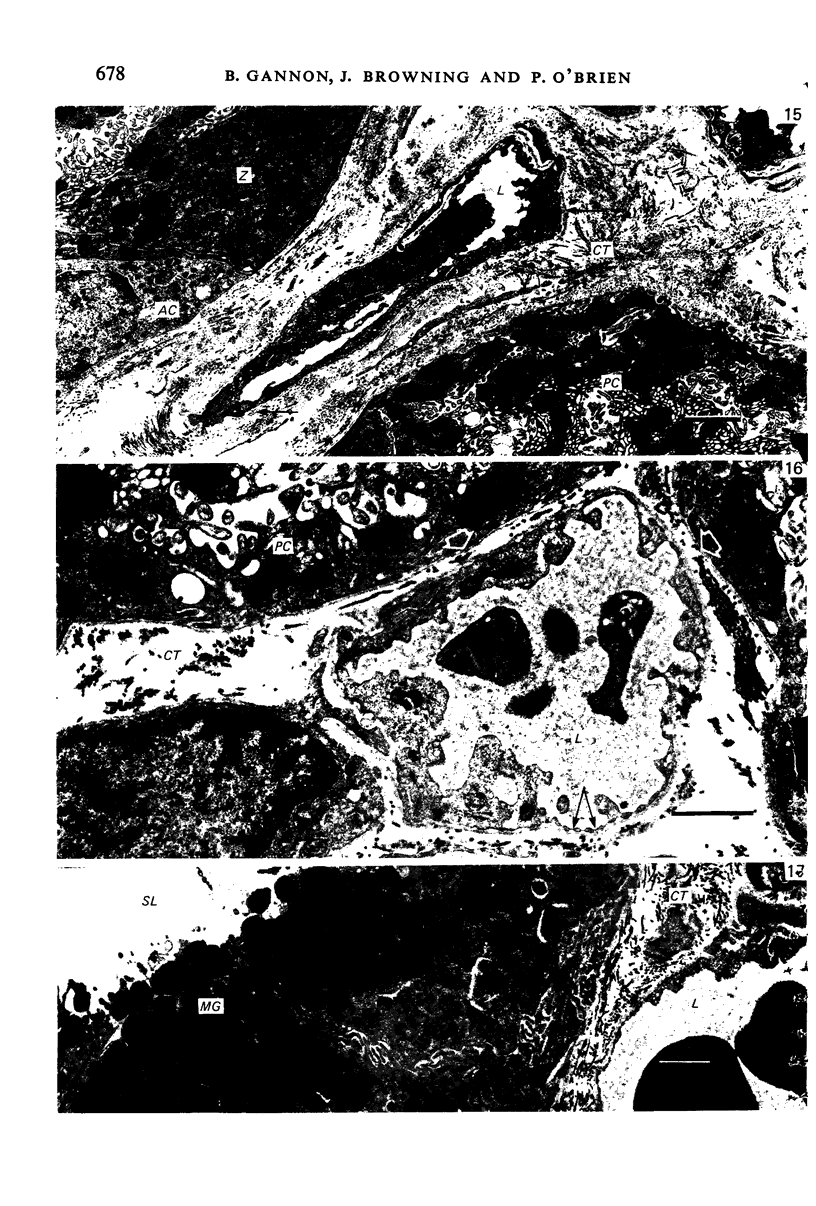
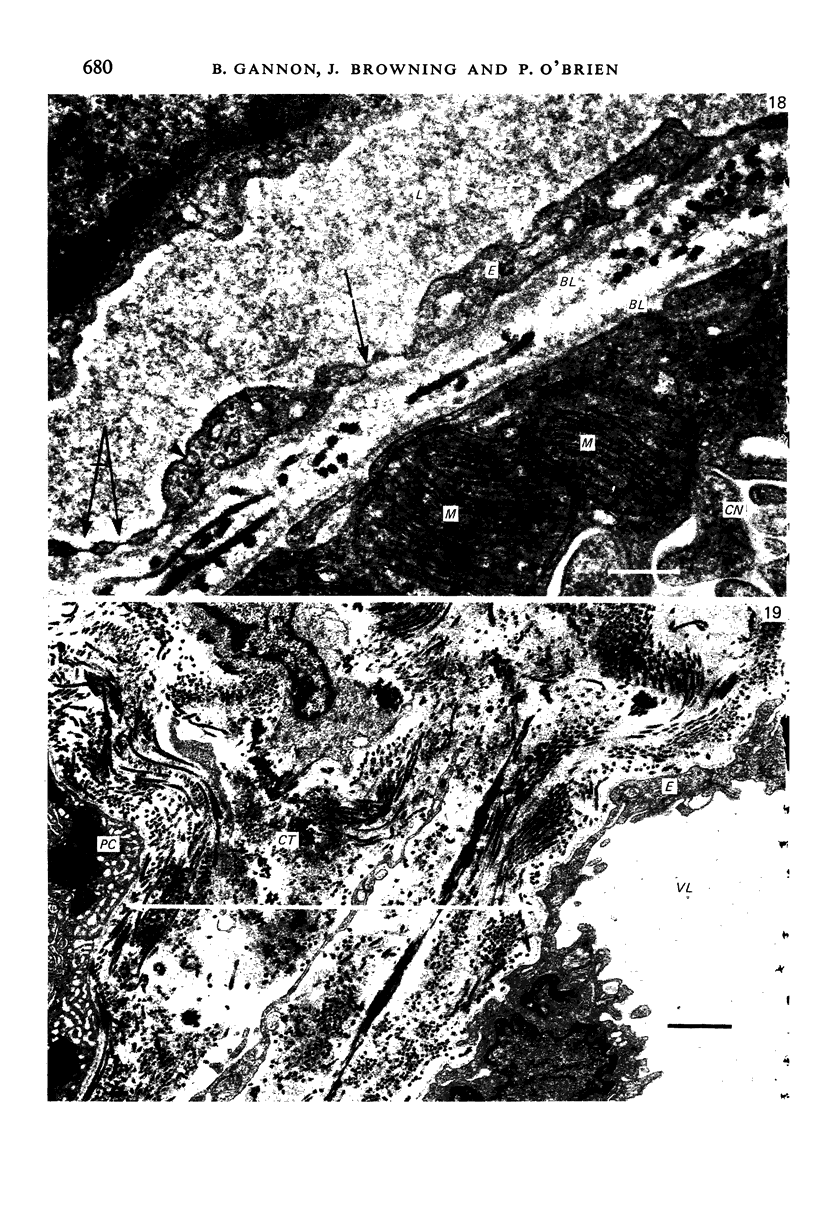
The circulatory pattern in the gastric mucosa of the rat and relationships of mucosal capillaries to gastric gland cells were investigated. Techniques used included the vascular corrosion cast/scanning electron microscope method, scanning electron microscopy of acid-digested tissues, conventional transmission electron microscopy and in vivo light microscopy. Arterial break-up into capillaries invariably occurs around the base of the gastric glands. The mucosal capillaries are fenestrated and vesiculated, and pass in close proximity to the abluminal aspects of the cells of the gastric glands, particularly the parietal cells. At the apices of the glands, the capillaries form a honeycomb network closely applied to the abluminal aspect of the surface epithelial cells, before draining into infrequent venules which are embedded in a substantial connective tissue sheath. No capillary drainage occurs into these venules deeper in the mucosa. No evidence of either mucosal or submucosal arteriovenous anastomoses was found. Because of the close proximity of the fenestrated mucosal capillaries to the parietal cells and surface epithelial cells and the direction of capillary blood flow, the alkaline tide of the actively secreting parietal cell must be transferred to the abluminal aspect of the surface epithelial cells. The capacity of these cells to secrete HCO3- or to neutralize back diffusing H+ ions would thereby be increased.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980 Mar;21(3):249–262. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW T. E., BENTLEY F. H., WALDER D. N. Arteries, veins, and arteriovenous anastomoses in the human stomach. Surg Gynecol Obstet. 1951 Dec;93(6):657–671. [PubMed] [Google Scholar]
- Bohlen H. G., Gore R. W. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976 Jan;11(1):103–110. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- Flemström G. Active alkalinization by amphibian gastric fundic mucosa in vitro. Am J Physiol. 1977 Jul;233(1):E1–12. doi: 10.1152/ajpendo.1977.233.1.E1. [DOI] [PubMed] [Google Scholar]
- Guth P. H., Rosenberg A. In vivo microscopy of the gastric microcirculation. Am J Dig Dis. 1972 May;17(5):391–398. doi: 10.1007/BF02231290. [DOI] [PubMed] [Google Scholar]
- Guth P. H., Smith E. Neural control of gastric mucosal blood flow in the rat. Gastroenterology. 1975 Oct;69(4):935–940. [PubMed] [Google Scholar]
- Hase T., Moss B. J. Microvascular changes of gastric mucosa in the development of stress ulcer in rats. Gastroenterology. 1973 Aug;65(2):224–234. [PubMed] [Google Scholar]
- Lanciault G., Jacobson E. D. The gastrointestinal circulation. Gastroenterology. 1976 Nov;71(5):851–873. [PubMed] [Google Scholar]
- NYLANDER G., OLERUD S. The vascular pattern of the gastric mucosa of the rat following vagotomy. Surg Gynecol Obstet. 1961 Apr;112:475–480. [PubMed] [Google Scholar]
- O'Brien P., Bushell M. Role of acid-base status in the response of the isolated amphibian gastric mucosa to back diffusion of H+. Gastroenterology. 1980 Sep;79(3):439–446. [PubMed] [Google Scholar]
- O'Brine P., Silen W. Influence of acid secretory state on the gastric mucosal tolerance to back diffusion of H+. Gastroenterology. 1976 Nov;71(5):760–765. [PubMed] [Google Scholar]
- Oka S. [Microcirculation of the gastro-intestinal mucosa and the architecture of the blood vessels]. Saishin Igaku. 1970 Aug;25(8):1705–1713. [PubMed] [Google Scholar]
- Pellegrini M. S. Sulla ultrastruttura dei capillari sanguigni in diverse zone della mucosa gastrica. Boll Soc Ital Biol Sper. 1968 Aug 15;44(15):1210–1211. [PubMed] [Google Scholar]
- Piasecki C. Blood supply to the human gastroduodenal mucosa with special reference to the ulcer-bearing areas. J Anat. 1974 Nov;118(Pt 2):295–335. [PMC free article] [PubMed] [Google Scholar]
- Piasecki C. Observations on the submucous plexus and mucosal arteries of the dog's stomach and first part of the duodenum. J Anat. 1975 Feb;119(Pt 1):133–148. [PMC free article] [PubMed] [Google Scholar]
- Robert A. Proposed terminology for the anatomy of the rat stomach. Gastroenterology. 1971 Feb;60(2):344–345. [PubMed] [Google Scholar]
- Rogers P. A., Gannon B. J. The vascular and microvascular anatomy of the rat uterus during the oestrous cycle. Aust J Exp Biol Med Sci. 1981 Dec;59(Pt 6):667–679. doi: 10.1038/icb.1981.59. [DOI] [PubMed] [Google Scholar]
- Rosenberg A., Guth P. H. A method for the in vivo study of the gastric microcirculation. Microvasc Res. 1970 Jan;2(1):111–112. doi: 10.1016/0026-2862(70)90056-7. [DOI] [PubMed] [Google Scholar]
- SCHNITZLEIN H. N. Regulation of blood flow through the stomach of the rat. Anat Rec. 1957 Apr;127(4):735–753. doi: 10.1002/ar.1091270409. [DOI] [PubMed] [Google Scholar]
- Smith P., O'Brien P., Fromm D., Silen W. Secretory state of gastric mucosa and resistance to injury by exogenous acid. Am J Surg. 1977 Jan;133(1):81–85. doi: 10.1016/0002-9610(77)90198-2. [DOI] [PubMed] [Google Scholar]
- TEORELL T. The acid-base balance of the secreting isolated gastric mucosa. J Physiol. 1951 Jul;114(3):267–276. doi: 10.1113/jphysiol.1951.sp004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Suyama K. Visualization of the adventitial aspect of the vascular smooth muscle cells under the scanning electron microscope. J Electron Microsc (Tokyo) 1978;27(2):157–159. [PubMed] [Google Scholar]
- Williams S. E., Turnberg L. A. Demonstration of a pH gradient across mucus adherent to rabbit gastric mucosa: evidence for a 'mucus-bicarbonate' barrier. Gut. 1981 Feb;22(2):94–96. doi: 10.1136/gut.22.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]