FIGURE 3.
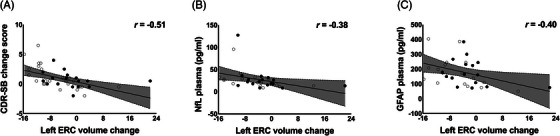
ERC volume change over 78 weeks of treatment in participants with MCI due to AD who are non‐carriers of APOE ε4 is associated with clinical cognitive change and blood plasma markers of neurodegeneration. The change in left ERC volume over 78‐week treatment showed a significant correlation with (A) change in the CDR‐SB score over 78 weeks (placebo: r = −0.69, P < 0.001; AGB101: r = −0.41, P = 0.12; overall: r = −0.51, P < 0.001), (B) NfL blood plasma levels at the end of study visit (placebo: r = −0.44, P = 0.10; AGB101: r = −0.47, P = 0.07; overall: −0.38, P < 0.05), and (C) GFAP blood plasma levels at the end of study visit (placebo: r = −0.39, P = 0.15; AGB101: r = −0.10, P = 0.72; overall: r = −0.40, P < 0.05). Open circles identify participants in the placebo condition and closed circles identify participants in the AGB101 condition. AD, Alzheimer's disease; APOE, apolipoprotein E; CDR‐SB, Clinical Dementia Rating Sum of Boxes score; ERC, entorhinal cortex; GFAP, glial fibrillary acidic protein; MCI, mild cognitive impairment; NfL, neurofilament light chain
