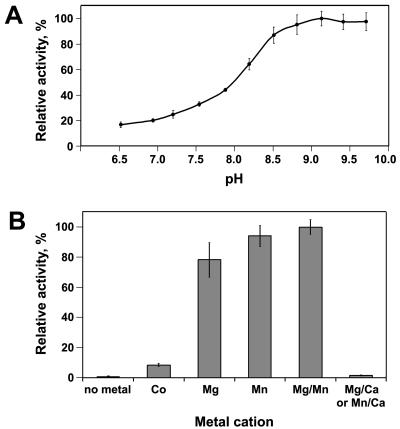
FIG. 3.
(A) pH dependence of the PDE-A activity of YahA. (B) Dependence of the PDE-A activity of YahA on divalent metal cations. The PDE-A reaction buffer (pH 9.35) contained different divalent metal cations (final concentration, 5 mM) instead of Mg2+. The bar on the right shows inhibition of the PDE-A activity in the standard reaction buffer containing 5 mM (final concentration) MgCl2 or 5 mM (final concentration) MnCl2 by Ca2+ (5 mM [final concentration] CaCl2). The reaction with Co2+ was carried out in PDE buffer at pH 8.4 to avoid precipitation of Co2+ complexes at higher pH values. The error bars indicate the standard deviations calculated from at least three replicates.