Abstract
Clostridium cellulovorans produces a major noncellulosomal family 9 endoglucanase EngO. A genomic DNA fragment (40 kb) containing engO and neighboring genes was cloned. The nucleotide sequence contained reading frames for endoglucanase EngO, a putative response regulator, and a putative sensor histidine kinase protein. The engO gene consists of 2,172 bp and encodes a protein of 724 amino acids with a molecular weight of 79,474. Northern hybridizations revealed that the engO gene is transcribed as a monocistronic 2.6-kb mRNA. 5′ RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) PCR analysis indicated that the single transcriptional start site of engO was located 264 bp upstream from the first nucleotide of the translation initiation codon. Alignment of the engO promoter region provided evidence for highly conserved sequences that exhibited strong similarity to the σA consensus promoter sequences of gram-positive bacteria. EngO contains a typical N-terminal signal peptide of 28 amino acid residues, followed by a 149-amino-acid sequence which is homologous to the family 4-9 carbohydrate-binding domain. Downstream of this domain was an immunoglobulin-like domain of 89 amino acids. The C terminus contains a family 9 catalytic domain of glycosyl hydrolase. Mass spectrometry analysis of EngO was in agreement with that deduced from the nucleotide sequence. Expression of engO mRNA increased from early to middle exponential phase and decreased during the early stationary phase. EngO was highly active toward carboxymethyl cellulose but showed no activity towards xylan. It was optimally active at 40 to 50°C and pH 5 to 6. The analysis of the products from the cellulose hydrolysis through thin-layer chromatography indicated its endoglucanase activity.
Clostridium cellulovorans ATCC 35296 (43) is a mesophilic, anaerobic, spore-forming bacterium which can utilize cellulose and other plant cell wall polysaccharides (9, 48). C. cellulovorans produces an extracellular enzyme complex (called a cellulosome) containing a variety of cellulolytic subunits attached to the nonenzymatic scaffolding protein CbpA (10, 39, 42). All cellulosomal enzymatic subunits contain a twice-repeated sequence called the dockerin domain that is lacking in noncellulosomal cellulolytic enzymes (1).
C. cellulovorans also produces noncellulosomal enzymes such as EngD (17), EngF (40), ArfA (28), and BgaA (28) that work synergistically with the cellulosomal enzymes (28). Thus far, 12 cellulosomal enzymatic subunits and 4 noncellulosomal enzymes from C. cellulovorans have been sequenced, including cellulases, xylanases, a mannanase, and a pectate lyase from eight different glycoside hydrolase families (9). Among the cellulosomal cellulase genes identified, five encode family 9 glycoside hydrolases, i.e., EngK (48), EngM (48), EngY (46), EngH (48), and EngL (45).
The nucleotide sequence, expression, and the characterization of the engO gene and its product EngO, a family 9 noncellulosomal endoglucanase from C. cellulovorans, are reported in this paper. A detailed transcriptional analysis of the engO promoter and its regulation during growth phase were carried out in order to gain some understanding of the expression pattern of this gene relative to the cellulosomal genes. The results indicate that EngO is a major noncellulosomal cellulase and that it is produced coordinately with the cellulosome. The analysis of its hydrolytic products indicates that it is an endoglucanase and produces primarily cellobiose. It is of interest that most of the cellulolytic cellulosomal and noncellulosomal enzymes produced by C. cellulovorans are members of glycosyl hydrolase family 9.
MATERIALS AND METHODS
Bacterial strains and fosmid.
C. cellulovorans ATCC 35296, used for isolation of the cellulosome fraction, was described previously (41). The Escherichia coli EPI300 (Epicentre) was used to propagate the fosmid library.
Fosmid library construction, screening, and DNA sequencing.
A C. cellulovorans fosmid library was constructed as described previously (19). The procedure yielded 3,000 recombinant clones. The Luria-Bertani (LB) plates, on which the recombinant E. coli were grown, were overlaid with soft agar containing 0.3% carboxymethyl cellulose (CMC; Sigma) and 0.7% agar in 25 mM sodium acetate buffer (pH 6.0). After incubation at 37°C for 16 h, the plates were stained with 0.3% Congo red and destained with 1 M NaCl. The clones that formed the halos were selected as CMCase-positive colonies. The CMCase-positive clones were picked and restreaked to confirm formation of clearing zones around the colonies on LB-CMC, followed by applying colony hybridization (Roche) according to the manufacturers' instructions with previously identified gene-specific probes such as cbpA, engE, engB, and engD (20, 22). The knockout (CMC-negative) clones were then isolated by using the EZ::TN <oriV/KAN-2> insertion system (Epicentre) (19). The transposon insertion sites of mutant clones were mapped by DNA sequencing with the transposon-specific flanking primers (Epicentre).
5′ RLM-RACE and Northern blot analysis.
The RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) and Northern blot technique were carried out with total RNA extracted from a C. cellulovorans culture grown on cellobiose and were used to determine the transcription start points and size of mRNA. Rapid amplification of 5′ cDNA ends was carried out using a FirstChoice RLM-RACE kit (Ambion) according to the manufacturer's instructions with the following exceptions. The nested PCR conditions for 5′ outer PCR were with 10 pmol gene-specific outer primer engO-5′-Outer (5′-TAAGCACGATAATCTCCACC), 1.25 units of Ambion's SuperTaq polymerase, 10 pmol 5′ RACE outer primer (5′-GCTGATGGCGATGAATGAACACTG) (Ambion), 1× SuperTaq PCR buffer (Ambion), 100 μM deoxynucleoside triphosphates, 1 ng/μl first-strand cDNA reaction, and H2O to 50 μl. The PCR conditions were as follows: (1×) 94°C, 4 min; (35×) 94°C, 30 s; 60°C, 30 s; 72°C, 40 s; and (1×) 72°C, 7 min. The 5′ inner PCR was carried out with 10 pmol gene-specific inner primer engO-5′-Inner (5′-CGTTGAAAAACTAGCATAACTTCCA) and 10 pmol 5′ RACE inner primer (5′-CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATG) (Ambion) using the same conditions as for the 5′ outer PCR. PCR products were observed on 2% agarose gels and sequenced. For Northern blotting, RNA samples (5 μg) were separated by 1% gel electrophoresis and blotted onto a nylon membrane (Ambion). The membrane was treated with engO probe (5′-AAAAGGGGAATAGTGATATG/5′-TAAGCACGATAATCTCCACC) or previously identified gene-specific probes (22) and detected as described previously (19).
Preparation of noncellulosomal proteins.
The noncellulosomal proteins were purified from culture supernatants of C. cellulovorans cells as described previously (41). The culture supernatants were obtained from the cultures at stationary phase (4 to 7 days) by centrifugation. The supernatants were precipitated by 80% (wt/vol) ammonium sulfate saturation and dialyzed. The extracellular material was then mixed with Avicel, which resulted in binding of the cellulosome complex to Avicel. After incubation for 1 h at 4°C, the suspension was poured into a column. The column was washed with 3 volumes of 50 mM Tris-HCl buffer (pH 7.5) to elute the unattached fractions as the noncellulosomal proteins. The unattached fraction was concentrated with Ultrafree Biomax (10-kDa cutoff; Millipore) before being subjected to gel filtration on a HiLoad 26/60 Superdex 200 prep grade column (320 ml; Amersham Biosciences) equilibrated with 50 mM Tris-HCl buffer (pH 7.5) by using the fast protein liquid chromatography system (Amersham Biosciences). The concentration of fractionated protein was measured by the method of Bradford (4) with a protein assay kit from Bio-Rad, using bovine serum albumin as the standard.
SDS-PAGE, zymogram, and mass spectrometry analysis.
The recombinant enzyme was isolated from culture supernatants of a CMCase-positive clone as described previously (19). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 10% polyacrylamide gel by the method of Laemmli (30). Proteins were fixed in the gels by soaking in a solution containing 40% (vol/vol) methanol and 10% (vol/vol) acetic acid for approximately 1 h and subsequently visualized by Coomassie blue staining (Genomic Solutions). The zymograms for CMCase were performed by using a 0.1% CMC (wt/vol) incorporated into the polyacrylamide. After SDS-PAGE, the gels were renatured in a renaturation buffer (100 mM succinic acid, 10 mM CaCl2, 1 mM dithiothreitol, pH 6.3) for 2 h at 25°C with gentle shaking. The renatured gel was then incubated in a fresh renaturation buffer for 1 h at 37°C with gentle shaking. The clearing zones corresponding to enzyme activities were visualized with 0.3% (wt/vol) Congo red (stained for 10 min and destained with 1 M NaCl solution) (2). Mass spectrometric analysis was performed to identify the engO on cellulosome, and recombinant proteins were separated by SDS-PAGE as described previously (22).
TLC.
Cellulose degradation products were determined by thin-layer chromatography (TLC) on precoated TLC sheets (silica gel; Whatman) with acetone-ethylacetate-acetic acid (2:1:1, vol/vol/vol) (32). The plates were visualized by spraying with a 1:1 (vol/vol) mixture of 0.2% methanolic orcinol and 20% sulfuric acid.
Nucleotide sequence accession number.
The nucleotide sequence reported here has been submitted to GenBank and can be accessed under accession number AY646113.
RESULTS AND DISCUSSION
Cloning and nucleotide sequence of the engO gene.
A previously constructed C. cellulovorans fosmid library (19) was screened for endoglucanase activity by overlaying with soft agar containing CMC, followed by colony hybridization analysis with probes made from previously identified genes such as cbpA (42), engE (47), engB (12), and engD (18). In this work, we located engO on the fosmid clone which contained a 50-kb insert DNA without any other endoglucanase or xylanase genes. The EZ::TN <oriV/KAN-2> insertion system (Epicentre), based on in vitro transposon 5 (16), was used for the generation of insertion knockout mutants of engO. The transposon insertion sites of mutant clones were mapped by nucleotide sequencing with the transposon-specific flanking primers (Epicentre). As a result of DNA sequencing, there is an open reading frame coding for an endoglucanase engO gene, which consists of 2,172 nucleotides encoding a protein of 724 amino acids with a predicted molecular weight of 79,473 (GenBank accession number AY646113). A putative ribosome-binding site, GGGGA, is present at a spacing 8 bp upstream of the translational start codon ATG.
In order to localize the promoter, the transcription start site of engO was determined by 5′ RLM-RACE PCR analysis described previously (19). A 336-bp PCR fragment (engO-5′-Inner/5′ RACE inner primer) was generated for DNA sequencing of the upstream region up to the transcription start site of engO. One clear ending sequence, corresponding to position 264 bp upstream from the A of the first ATG, was obtained by DNA sequencing of the 5′ RLM-RACE PCR product. The engO mRNA start point suggested a putative promoter sequence, TTGCAA and TAATAT, with a 17-bp spacing between them (Fig. 1).
FIG. 1.
Alignment of putative engO promoter located at −10 and −35 bases upstream of the start point of transcription. Transcription start points are indicated by the bent arrows. The nucleotide numbering begins from the first codon shown on the right. The consensus (Cons) sequence derived from this alignment is given at the bottom. It consists of nucleotides that are present in any given position in more than 50% of the sequences. Promoter sequence nucleotides that match those of the consensus sequence are in black boxes. The coordinates refer to the published nucleotide sequences: cbpA (42), GenBank accession no. M73817; engE (47), GenBank accession no. AF105331; xynB (19), GenBank accession no. AY604045; and manA (45), GenBank accession no. AF132735.
The consensus sequence of engO and previously determined C. cellulovorans promoters (19, 21), at −10 (5′-TTGAAA) and at −35 (5′-TATAAT), are highly homologous to elements recognized by Bacillus subtilis (σA) and E. coli (σ70) RNA polymerases (36, 50) (Fig. 1). The spacing between the −35 and −10 regions was 17 bp, which is the optimal spacing observed for B. subtilis and E. coli promoter sequences (6, 23). The close similarity of the promoter to the consensus σA sequence suggests that the engO promoter, if not subjected to any regulatory constraints, would act as a strong promoter in vivo (8, 24).
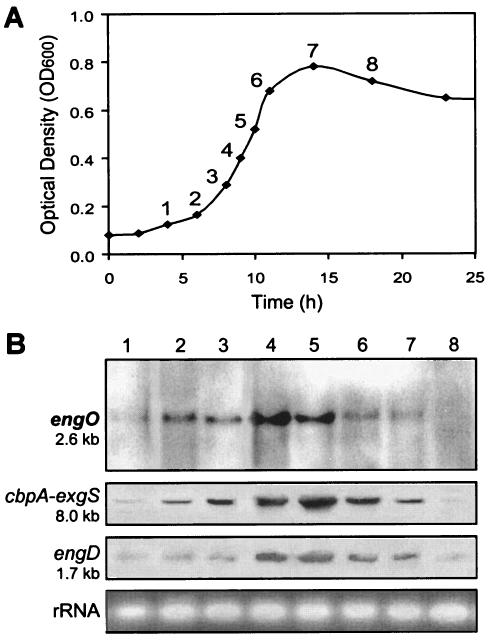
Northern hybridizations of the RNA with an engO probe showed a single transcript of 2.6 kb (Fig. 2B). This mRNA was in good agreement with the size of the engO gene (2,172 bp) and indicated that engO was a monocistronic gene. On the basis of the size of engO mRNA and the location of the transcription start site, a putative transcription terminator that consists of a 47-bp palindromic sequence, corresponding to an mRNA hairpin loop with a ΔG of −72.0 kcal/mol (5, 44), was found downstream of the TAA termination codon at nucleotide position 3665 (GenBank accession number AY646113). This structure is similar to the rho factor-independent terminator of E. coli (36).
FIG. 2.
Relative levels of transcripts of engO at different growth phases of cellobiose cultures. (A) C. cellulovorans growth curve. (B) Northern blot analyses were conducted with the same concentrations of RNA (5 μg) isolated from C. cellulovorans culture grown on 0.5% cellobiose as the sole carbon source. Ethidium bromide staining of rRNA is shown as a loading control. The numbers of the lanes in panel B represent the numbers of the growth curve points in panel A.
Transcriptional analysis of engO.
To determine whether the engO along with C. cellulovorans genes are regulated coordinately, changes in the expression level of mRNA were monitored during the cultivation of C. cellulovorans on 0.5% cellobiose as the sole carbon source. RNA was prepared from the culture at different stages of growth. The RNA was subjected to Northern blot analyses using primers that were specific to the engO, cbpA-exgS, and engD genes. These genes represent the major cellulosomal subunits (cbpA-exgS) (31, 42) and a noncellulosomal endoglucanase gene, engD (17). A semiquantitative measure of the level of engO mRNA, using digoxigenin-labeled probes and RNA isolated at different times during cell growth, was obtained by Northern blot analysis (Fig. 2A and B). The levels of engO mRNA increased simultaneously from early to middle exponential phase and dramatically decreased during the early stationary phase when the cells were grown on cellobiose (Fig. 2A and B, lanes 1 to 8). Like engO gene expression, cells contained high levels of cbpA, exgS, and engD mRNAs during most of the exponential growth phase with the level being the highest at the middle of the exponential phase (Fig. 2A and B). The mRNA of engO along with those of cbpA-exgS and xynA were coordinately expressed.
Amino acid sequence analysis of EngO.
Comparison of the amino acid sequence of EngO with those registered in SWISS PROT and GenBank databases clearly revealed that the mature EngO consisted of three distinct functional domains. The deduced N-terminal sequence of 28 amino acids contains a sequence similar to the signal peptide sequences found in prokaryotic secretory proteins (33). The N-terminal domain of the mature form of EngO, 149 amino acid residues downstream of the signal peptide, is homologous with the family 4 (subfamily 9) carbohydrate-binding domain (CBM 4-9) of other endoglucanases, i.e., 27% sequence identity with CelN of Clostridium thermocellum (52), 27% identity with EgVII of Ruminococcus albus (34), 26% identity with LamA of Thermotoga neapolitana (51), and 26% identity with CenC of Cellulomonas fimi (7).
The N-terminal immunoglobulin (Ig)-like domain of EngO, extending from position 190 to 278, exhibited 40% sequence identity with CelE of Clostridium cellulolyticum (14), 38% identity with CelD of Cytophaga hutchinsonii (GenBank access number ZP_00307924), 32% identity with Ced1 of Butyrivibrio fibrisolvens (3), and 32% identity with CelD of Microbulbifer degradans (GenBank access number ZP_00315637). Some enzymes in family 9 cellulases show a tight association of an Ig domain with the catalytic domain. For example, EngK and EngM of C. cellulovorans contain an Ig-like domain at the N-terminal side of the catalytic domain. In the earlier classification of cellulases, the enzymes in family 9 of the present classification had been compiled into two subfamilies, on the basis of amino acid sequence similarities (35). Most enzymes in one subfamily show a tight association of an Ig-like domain with a catalytic domain; e.g., EngK and EngM of C. cellulovorans and CbhA, CelD, CelJ, and CelK of C. thermocellum (29) contain an Ig-like domain at the N-terminal side of the catalytic domain.
The catalytic domain in EngO is a family 9 glycosyl hydrolase domain (435 amino acid residues) located in the C terminus of the peptide, with 38% sequence identity with CelA of Alicyclobacillus acidocaldarius (11), 36% identity with Ced1 of B. fibrisolvens (3), 35% identity with CelD of C. thermocellum (25), and 35% identity with EGC of Fibrobacter succinogenes (GenBank access number AAC41523). The catalytic domains of these enzymes are highly conserved in nine regions and presumably serve to form the overall structure of these enzymes, suggesting that these endoglucanases have diverged from a common evolutionary ancestor. Family 9 cellulases catalyze the hydrolysis of β-1,4 linkages with the inversion of anomeric carbon configuration. The structure of the catalytic domain in this family consists of an (α/α)6-helical barrel (26, 37). Two aspartic acids, a histidine, and a glutamic acid, which have been confirmed as the catalytic amino acids in C. thermocellum (49), were conserved in four identical regions as the nucleophile and the proton donor.
The domain organization of EngO (CBM4-Ig-GH9) compared with those of closely related cellulases such as C. cellulolyticum CelE (14), C. thermocellum CelK (27), and Streptomyces reticuli Cel1 (38) reveals that conservation among catalytic cores is greater than conservation among CBM4, indicating either a lower level of evolutionary pressure on these last domains or changes in function and specificities. In addition, EngO exhibits a common domain organization among family 9 endoglucanases such as EngK and EngM in C. cellulovorans, sharing the same modular structure except lacking the dockerin domain as a noncellulosomal enzyme.
Identification and characterization of EngO as a recombinant protein.
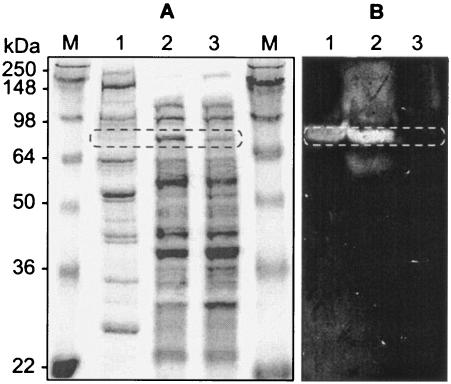
The product of engO was recovered from the supernatant of E. coli EPI300 grown on LB plates by ammonium sulfate precipitation. The noncellulosomal protein EngO was purified from C. cellulovorans grown on Avicel-containing medium in a cellulose affinity column (HiLoad 26/60 Superdex 200 prep grade column) as described previously (22). We analyzed the recombinant rEngO protein by zymograms and compared it with the native noncellulosomal EngO. The rEngO gave one major band in the zymogram with CMC, and the molecular mass of the enzyme was estimated to be 80 kDa (Fig. 3B, lane 2), which is similar in size to that of the mature native EngO deduced from the nucleotide sequence (79,474 Da). A band with an apparent molecular mass of 80 kDa was detected in the noncellulosomal proteins purified from C. cellulovorans (Fig. 3A and B, lanes 1). The size of the protein was in good agreement with that of the full-length EngO produced by recombinant E. coli and the size calculated from the deduced amino acid sequence. The mass spectrometry technique (22) was also used for the identification of proteins separated by SDS-PAGE. The mass spectra of EngO showed the amino acid sequences of tryptic peptides AYVDGQINTTSAVQSISK, AAFGDDFNIPESGNGVSDVLDEVK, NSLGFSFVSGYGTDYLK, and VVSTATNEVVYTGNIEGCR, which perfectly matched the deduced sequence of EngO. The tryptic peptides were found on the deduced entire (N- to C-terminal) sequences of EngO. The profiles based on SDS-PAGE, mass spectrometry, and zymogram analysis suggest that EngO is the major noncellulosomal endoglucanase.
FIG. 3.
Expression of EngO in C. cellulovorans and E. coli. (A) Gels stained with Coomassie blue. (B) Gels stained for CMCase activity. Lanes: M, protein mass standards; 1, noncellulosomal proteins of C. cellulovorans; 2, supernatant of recombinant E. coli; 3, supernatant of knockout EngO recombinant E. coli. The oblong circles represent the putative EngO (79,474 Da).
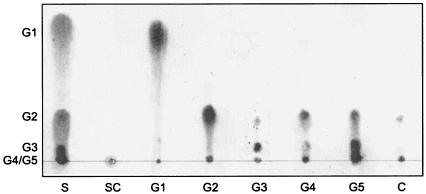
The optimum temperature for activity was found to be 40 to 50°C at pH 6 (data not shown). The optimum pH for activity was found to be pH 5 to 6 when the enzyme activity was assayed at 37°C in sodium acetate buffer solutions at various pHs (data not shown). TLC analysis of the soluble sugars released from cellooligosaccharides indicated that the main product was mainly cellobiose and cellotriose, with cellotetraose and glucose as minor products (Fig. 4). Although the main product from CMC was cellobiose, cellotriose was also detected. This enzyme was less active toward cellotriose and inactive toward cellobiose. The general characteristics of EngO, including temperature and pH for maximum activity, are similar to family 9 cellulosomal endoglucanases such as EngK, EngM, and EngY of C. cellulovorans (T. Arai and R. H. Doi, unpublished data). In addition, TLC analysis with cellooligosaccharides showed that all the cellooligosaccharides (cellotriose, cellotetraose, and cellopentaose) were hydrolyzed except for cellobiose, suggesting that EngO hydrolyzes CMC in a manner typical of many endoglucanases (13, 15). In conclusion, the engO of C. cellulovorans was found to encode a novel major family 9 noncellulosomal cellulase. Further studies should reveal whether EngO, as a family 9 noncellulosomal endoglucanase, works synergistically with the cellulosome to degrade the cellulose present in plant cell walls.
FIG. 4.
Thin-layer chromatography of hydrolysis products from oligosaccharides and CMC. The oligosaccharides and CMC (1 mg) were incubated with enzyme (1 μg) for 16 h, and the hydrolysates were analyzed by thin-layer chromatography. S, authentic oligosaccharides; SC, CMC reaction mixture with knockout recombinant EngO; G1, glucose; G2, cellobiose; G3, cellotriose; G4, cellotetraose; G5, cellopentaose; C, CMC.
Acknowledgments
This research was supported in part by a grant from the Research Institute of Innovative Technology for the Earth (RITE) and by U.S. Department of Energy grant DDF03-92ER20069.
REFERENCES
- 1.Bayer, E. A., J. P. Belaich, Y. Shoham, and R. Lamed. 2004. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58:521-554. [DOI] [PubMed] [Google Scholar]
- 2.Bequin, P. 1983. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal. Biochem. 131:333-336. [DOI] [PubMed] [Google Scholar]
- 3.Berger, E., W. A. Jones, D. T. Jones, and D. R. Woods. 1990. Sequencing and expression of a cellodextrinase (ced1) gene from Butyrivibrio fibrisolvens H17c cloned in Escherichia coli. Mol. Gen. Genet. 223:310-318. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Breslauer, K. J., R. Frank, H. Blocker, and L. A. Marky. 1986. Predicting DNA duplex stability from the base sequence. Proc. Natl. Acad. Sci. USA 83:3746-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, B. Y., Y. T. Shyu, and R. H. Doi. 1992. The interaction between Bacillus subtilis sigma-A (σA) factor and RNA polymerase with promoters. Biochimie 74:601-612. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho, J. B., B. Moser, D. G. Kilburn, R. A. Warren, and R. C. Miller, Jr. 1991. Nucleotide sequence of the endoglucanase C gene (cenC) of Cellulomonas fimi, its high-level expression in Escherichia coli, and characterization of its products. Mol. Microbiol. 5:1221-1233. [DOI] [PubMed] [Google Scholar]
- 8.Doi, R. H. 1991. Regulation of gene expression, p. 15-39. In U. N. Streips and R. E. Yasbin (ed.), Modern microbial genetics. Wiley-Liss, New York, N.Y.
- 9.Doi, R. H., A. Kosugi, K. Murashima, Y. Tamaru, and S. O. Han. 2003. Cellulosomes from mesophilic bacteria. J. Bacteriol. 185:5907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi, R. H., and Y. Tamaru. 2001. The Clostridium cellulovorans cellulosome: an enzyme complex with plant cell wall degrading activity. Chem. Rec. 1:24-32. [DOI] [PubMed] [Google Scholar]
- 11.Eckert, K., F. Zielinski, L. Lo Leggio, and E. Schneider. 2002. Gene cloning, sequencing, and characterization of a family 9 endoglucanase (CelA) with an unusual pattern of activity from the thermoacidophile Alicyclobacillus acidocaldarius ATCC27009. Appl. Microbiol. Biotechnol. 60:428-436. [DOI] [PubMed] [Google Scholar]
- 12.Foong, F., T. Hamamoto, O. Shoseyov, and R. H. Doi. 1991. Nucleotide sequence and characteristics of endoglucanase gene engB from Clostridium cellulovorans. J. Gen. Microbiol. 137:1729-1736. [DOI] [PubMed] [Google Scholar]
- 13.Gal, L., C. Gaudin, A. Belaich, S. Pages, C. Tardif, and J. P. Belaich. 1997. CelG from Clostridium cellulolyticum: a multidomain endoglucanase acting efficiently on crystalline cellulose. J. Bacteriol. 179:6595-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudin, C., A. Belaich, S. Champ, and J.-P. Belaich. 2000. CelE, a multidomain cellulase from Clostridium cellulolyticum: a key enzyme in the cellulosome? J. Bacteriol. 182:1910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilad, R., L. Rabinovich, S. Yaron, E. A. Bayer, R. Lamed, H. J. Gilbert, and Y. Shoham. 2003. CelI, a noncellulosomal family 9 enzyme from Clostridium thermocellum, is a processive endoglucanase that degrades crystalline cellulose. J. Bacteriol. 185:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goryshin, I. Y., and W. S. Reznikoff. 1998. Tn5 in vitro transposition. J. Biol. Chem. 273:7367-7374. [DOI] [PubMed] [Google Scholar]
- 17.Hamamoto, T., F. Foong, O. Shoseyov, and R. H. Doi. 1992. Analysis of functional domains of endoglucanases from Clostridium cellulovorans by gene cloning, nucleotide sequencing and chimeric protein construction. Mol. Gen. Genet. 231:472-479. [DOI] [PubMed] [Google Scholar]
- 18.Hamamoto, T., O. Shoseyov, F. Foong, and R. H. Doi. 1990. A Clostridium cellulovorans gene, engD, codes for both endo-β-1,4-glucanase and cellobiosidase activities. FEMS Microbiol. Lett. 72:285-288. [Google Scholar]
- 19.Han, S. O., H. Yukawa, M. Inui, and R. H. Doi. 2004. Isolation and expression of the xynB gene and its product XynB, a consistent component of the Clostridium cellulovorans cellulosome. J. Bacteriol. 186:8347-8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han, S. O., H. Yukawa, M. Inui, and R. H. Doi. 2003. Regulation of expression of cellulosomal cellulase and hemicellulase genes in Clostridium cellulovorans. J. Bacteriol. 185:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han, S. O., H. Yukawa, M. Inui, and R. H. Doi. 2003. Transcription of Clostridium cellulovorans cellulosomal cellulase and hemicellulase genes. J. Bacteriol. 185:2520-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansmeier, N., F. W. Bartels, R. Ros, D. Anselmetti, A. Tauch, A. Puhler, and J. Kalinowski. 2004. Classification of hyper-variable Corynebacterium glutamicum surface-layer proteins by sequence analyses and atomic force microscopy. J. Biotechnol. 112:177-193. [DOI] [PubMed] [Google Scholar]
- 23.Harley, C. B., and R. P. Reynolds. 1987. Analysis of E. coli promoter sequences. Nucleic Acids Res. 15:2343-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joliff, G., P. Beguin, and J. P. Aubert. 1986. Nucleotide sequence of the cellulase gene celD encoding endoglucanase D of Clostridium thermocellum. Nucleic Acids Res. 14:8605-8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juy, M., A. G. Amit, P. M. Alzari, R. J. Poljak, M. Claeyssens, P. Béguin, and J.-P. Aubert. 1992. Three-dimensional structure of a thermostable bacterial cellulase. Nature 357:89-91. [Google Scholar]
- 27.Kataeva, I. A., R. D. Seidel III, X. L. Li, and L. G. Ljungdahl. 2001. Properties and mutation analysis of the CelK cellulose-binding domain from the Clostridium thermocellum cellulosome. J. Bacteriol. 183:1552-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosugi, A., K. Murashima, and R. H. Doi. 2002. Characterization of two noncellulosomal subunits, ArfA and BgaA, from Clostridium cellulovorans that cooperate with the cellulosome in plant cell wall degradation. J. Bacteriol. 184:6859-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurokawa, J., E. Hemjinda, T. Arai, T. Kimura, K. Sakka, and K. Ohmiya. 2002. Clostridium thermocellum cellulase CelT, a family 9 endoglucanase without an Ig-like domain or family 3c carbohydrate-binding module. Appl. Microbiol. Biotechnol. 59:455-461. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Liu, C. C., and R. H. Doi. 1998. Properties of exgS, a gene for a major subunit of the Clostridium cellulovorans cellulosome. Gene 211:39-47. [DOI] [PubMed] [Google Scholar]
- 32.Morag, E., E. A. Bayer, and R. Lamed. 1990. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J. Bacteriol. 172:6098-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 34.Ohara, H., S. Karita, T. Kimura, K. Sakka, and K. Ohmiya. 2000. Characterization of the cellulolytic complex (cellulosome) from Ruminococcus albus. Biosci. Biotechnol. Biochem. 64:254-260. [DOI] [PubMed] [Google Scholar]
- 35.Ohmiya, K., K. Sakka, S. Karita, and T. Kimura. 1997. Structure of cellulases and their applications. Biotechnol. Genet. Eng. Rev. 14:365-414. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg, M., and D. Court. 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13:319-353. [DOI] [PubMed] [Google Scholar]
- 37.Sakon, J., D. Irwin, D. B. Wilson, and P. A. Karplus. 1997. Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat. Struct. Biol. 4:810-818. [DOI] [PubMed] [Google Scholar]
- 38.Schlochtermeier, A., S. Walter, J. Schroder, M. Moorman, and H. Schrempf. 1992. The gene encoding the cellulase (avicelase) Cel1 from Streptomyces reticuli and analysis of protein domains. Mol. Microbiol. 6:3611-3621. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 40.Sheweita, S. A., A. Ichi-ishi, J. S. Park, C. Liu, L. M. Malburg, Jr., and R. H. Doi. 1996. Characterization of engF, a gene for a non-cellulosomal Clostridium cellulovorans endoglucanase. Gene 182:163-167. [DOI] [PubMed] [Google Scholar]
- 41.Shoseyov, O., and R. H. Doi. 1990. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc. Natl. Acad. Sci. USA 87:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoseyov, O., M. Takagi, M. A. Goldstein, and R. H. Doi. 1992. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc. Natl. Acad. Sci. USA 89:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sleat, R., R. A. Mah, and R. Robinson. 1984. Isolation and characterization of an anaerobic, celluloytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 48:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugimoto, N., S. Nakano, M. Katoh, A. Matsumura, H. Nakamuta, T. Ohmichi, M. Yoneyama, and M. Sasaki. 1995. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry 34:11211-11216. [DOI] [PubMed] [Google Scholar]
- 45.Tamaru, Y., and R. H. Doi. 2000. The engL gene cluster of Clostridium cellulovorans contains a gene for cellulosomal manA. J. Bacteriol. 182:244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamaru, Y., and R. H. Doi. 2001. Pectate lyase A, an enzymatic subunit of the Clostridium cellulovorans cellulosome. Proc. Natl. Acad. Sci. USA 98:4125-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamaru, Y., and R. H. Doi. 1999. Three surface layer homology domains at the N terminus of the Clostridium cellulovorans major cellulosomal subunit EngE. J. Bacteriol. 181:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamaru, Y., S. Karita, A. Ibrahim, H. Chan, and R. H. Doi. 2000. A large gene cluster for the Clostridium cellulovorans cellulosome. J. Bacteriol. 182:5906-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomme, P., J. van Beeumen, and M. Claeyssens. 1992. Modification of catalytically important carboxy residues in endoglucanase D from Clostridium thermocellum. Biochem. J. 285:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young, M., N. P. Minton, and W. L. Staudenbauer. 1989. Recent advances in the genetics of the clostridia. FEMS Microbiol. Rev. 5:301-325. [DOI] [PubMed] [Google Scholar]
- 51.Zverlov, V. V., K. Piotukh, O. Dakhova, G. Velikodvorskaya, and R. Borriss. 1996. The multidomain xylanase A of the hyperthermophilic bacterium Thermotoga neapolitana is extremely thermoresistant. Appl. Microbiol. Biotechnol. 45:245-247. [DOI] [PubMed] [Google Scholar]
- 52.Zverlov, V. V., G. A. Velikodvorskaya, and W. H. Schwarz. 2003. Two new cellulosome components encoded downstream of celI in the genome of Clostridium thermocellum: the non-processive endoglucanase CelN and the possibly structural protein CseP. Microbiology 149:515-524. [DOI] [PubMed] [Google Scholar]