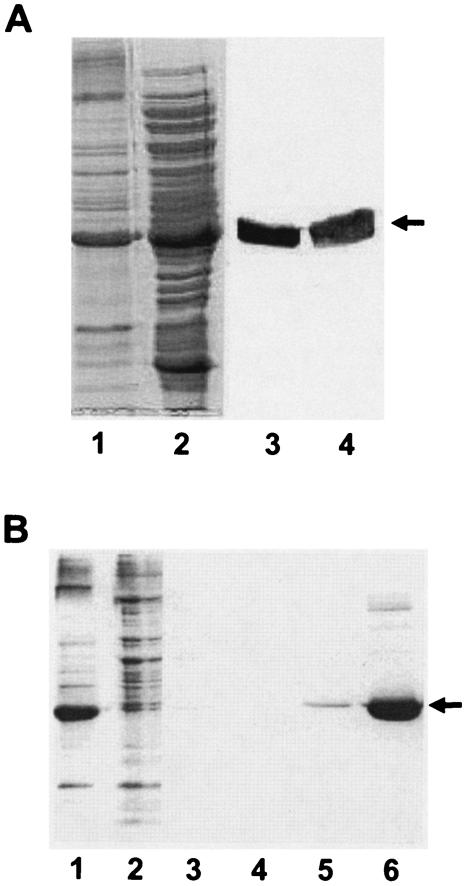
FIG. 2.
(A) Localization of Anabaena KdpD(6His) in E. coli cell fractions. Membranes and cytosolic fractions of BL21(DE3)/pLysS/pETDI were prepared as described in Materials and Methods. For comparison, the membrane pellet obtained after the ultracentrifugation step was resuspended (in lysis buffer) in a volume equal to that of the supernatant (cytosol). Lane 1, membrane vesicles (20 μl); lane 2, cytosol (20 μl). The samples were also electroblotted onto a nitrocellulose membrane and probed with the monoclonal anti-His antibody. Detection was carried out by a secondary anti-mouse immunoglobulin G coupled to alkaline phosphatase (lanes 3 and 4). Anabaena KdpD(His6) is indicated by an arrow. (B) Purification of Anabaena KdpD(6His) from E. coli membranes. Anabaena KdpD(6His) was solubilized with 1% Aminoxid WS35 from E. coli BL21(DE3)/pLysS/pETDI membranes and purified by affinity chromatography on an Ni-NTA matrix. The samples were resolved on an SDS-polyacrylamide (11%) gel and stained with Coomassie brilliant blue. Lane 1, solubilized membranes (10 μg); lane 2, unbound fraction (10 μg); lane 3, 10 mM imidazole wash; lane 4, 20 mM imidazole wash; lane 5, 40 mM imidazole wash; lane 6, 200 mM imidazole elution (5 μg). The position of Anabaena KdpD(His6) is indicated by an arrow.