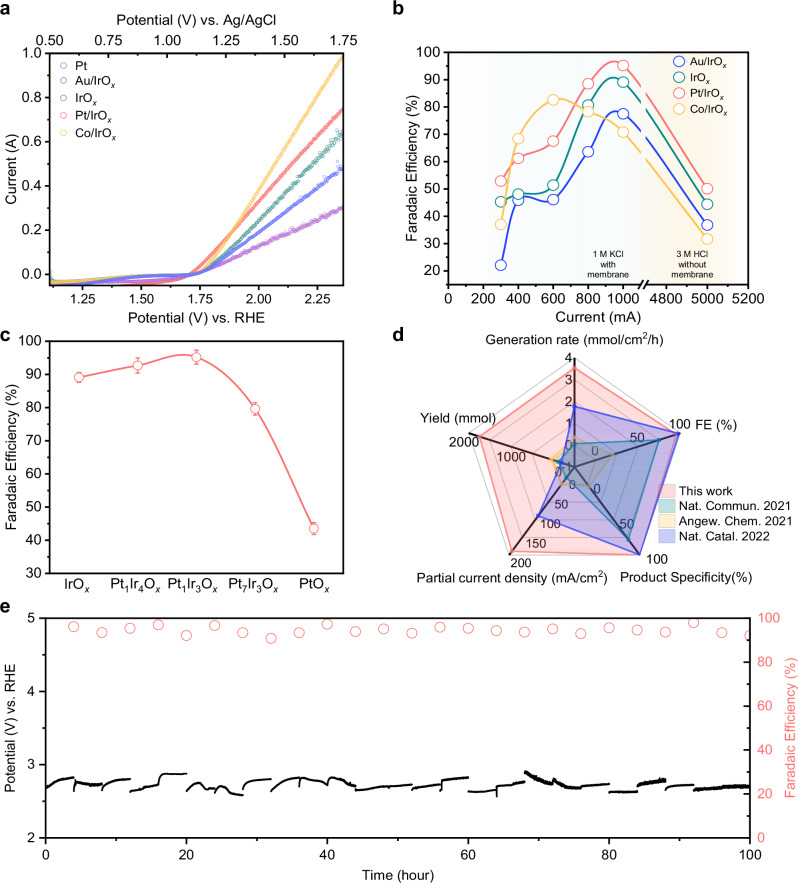
Fig. 5. Electrochemical chlorocyclohexane production of Ir-based electrocatalysts.
a LSV curves in 1.0 M KCl electrolyte with cyclohexane with various electrocatalysts. (Pt could be oxidized into PtOx during the halogenation process) No IR correction was conducted. b Chlorocyclohexane FE under different applied currents for each of the Ir-based electrocatalysts. Note: quantification was based on GCMS and the geometric area of electrode used in all cases was 5 cm2. c FE of chlorocyclohexane under current of 1 A (200 mA/cm2) of different catalysts with different ratio of Ir and Pt. d Comparison of partial current density, product generation rate, yield over stability, Faradaic efficiency, and product specificity with previous literature reports. Product specificity refers to the percentage of all reacted substrate going towards the desired product. e Applied potential and chlorocyclohexane FE for 100 h at 1 A for Pt/IrOx. After each cycle (4 h), the electrolyte was removed for product quantification and refreshed (total 25 cycles). Note: for each cycle, 10 ml of fresh cyclohexane was used. No IR correction was conducted. Source data are provided as a Source Data file.