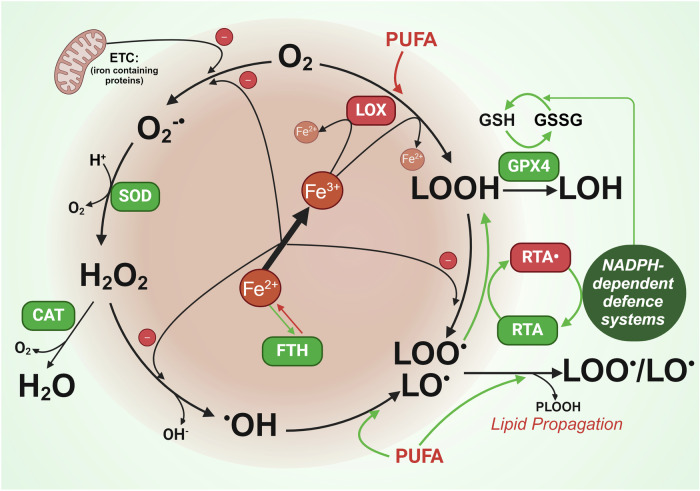
Fig. 1.
Central role of iron in reactive oxygen species generation and lipid peroxidation. Iron is involved directly and indirectly at several points to produce reactive oxygen species and lipid peroxidation. Indirectly, iron containing proteins in the electron transport chain (ETC) generate O2-• which is reduced to H2O2 by superoxide dismutase (SOD). H2O2 can either be quenched by catalase (CAT) or react with iron via the Fenton reaction, to generate hydroxyl radicals (•OH). The Fenton reaction can also catalyse the production of lipid peroxyl radicals (LOO• /LO•) from lipid hydroperoxide (LOOH). Radical trapping agents (RTAs) can quench lipid peroxyl radicals. Indirectly, iron contained in lipoxygenases (LOX) catalyse oxygenation of polyunsaturated fatty acids (PUFAs) and lipids to produce lipid hydroperoxide (LOOH). Glutathione peroxidase 4 (GPX4) can siphon lipid hydroperoxides away from fuelling lipid peroxidation and propagation by reducing PLOOH (high ferroptosis risk) to benign lipid alcohols (LOH). The reducing power of GPX4 is fuelled by reduced glutathione (GSH) which is dependent on NADPH to be recycled from its reduced form glutathione disulfide (GSSG). The breakdown of iron storage protein ferritin (FTH) can result in increased labile iron to facilitate these reactions. Figure created using Biorender.coms