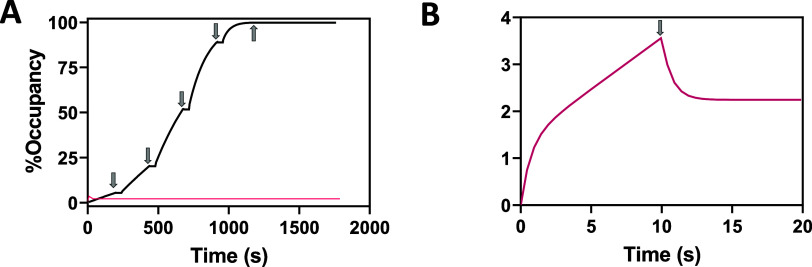
Figure 1.
Simulations facilitating the interpretation of SPR curves for characterization of covalent inhibitors binding to an immobilized target. Arrows indicate the end of each injection, which is followed by continuous buffer flow, allowing a dissociation phase to be measured. A response of 1 RU = 1 pg/mm2 and here is expressed in terms of protein occupancy, where % Occupancy = (Response/Saturation Response). Curves were simulated by numerical integration of coupled eqs 2 and 4 assuming rate constants kon = 2.2 × 105 M–1 s–1, koff = 1.27 s–1, kf= kinact = 0.15 s–1 and kr = 0 s–1. (A) Simulated binding/alkylation curve (black) for exposure of an irreversible covalent inhibitor to surface-bound protein. Repeated injections of inhibitor are simulated over five serial-tripling concentrations to a maximum concentration of 1 μM. (B) Simulated binding/alkylation curve where a single concentration (100 nM) of compound was injected for 10 s thereby limiting occupancy to <4% which resolves affinity binding (i.e., rapid rise/fall at start/stop of exposure) from accumulation of adduct (linear association segment and irreversible baseline increase). Note that this curve is also plotted in A (red) for comparison.