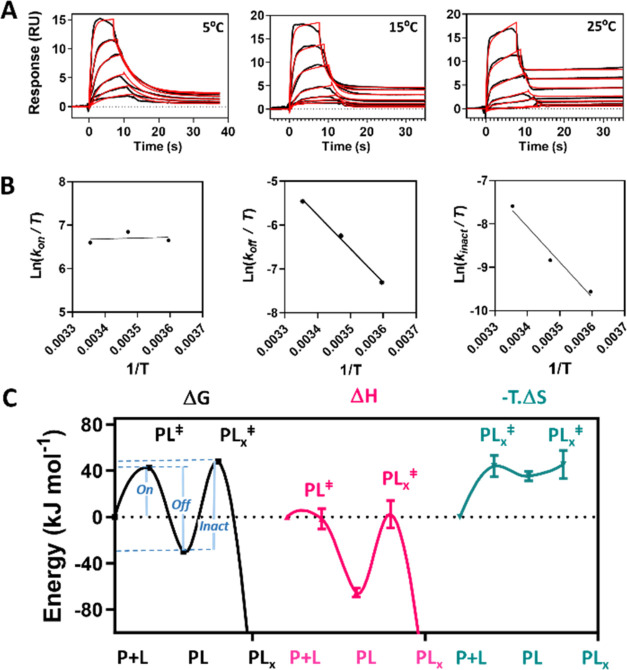
Figure 4.
Kinetic characterization of compound A at three temperatures and associated thermodynamic analysis. (A) SPR sensograms at various temperatures showing binding a serial doubling dose–response series of compound A from 5 μM with variable contact times (black) with respect to concentration. Reaction (3) was fitted to the data (red) through numerical integration of coupled eqs 3 and 4 assuming pseudo-first-order reaction kinetics. The relative SE associated with each fitted parameter were <0.6% other than for koff, where the maximum relative SE was 7.2%. All χ2 < 0.1 RU. (B) Eyring transition state model was fitted to the fundamental kinetic constants obtained from analysis in (A) with error bars indicating ± SE of the fit obtained in (A). (C) Energy transitions in ΔG⧧, ΔH⧧ and TΔS⧧ at 25 °C (chosen standard temperature) for compound A binding KRAS G12C on the reaction coordinate. The free energy barrier associated with each transition state is defined by the energy difference between the reactants, indicated along the reaction coordinate (x-axis) and the corresponding transition state, designated with superscript (⧧). The error bars represent the SE of the fit associated with each thermodynamic quantity returned from the fit in (B). The correspondence between transition state free energy ΔG⧧ and the fundamental kinetic rate constants are indicated by on, off and inact, respectively. The relative SE was insignificant (<1%) for estimation of ΔG⧧ but higher (see visible error bars) for its components ΔH⧧ and TΔS⧧.