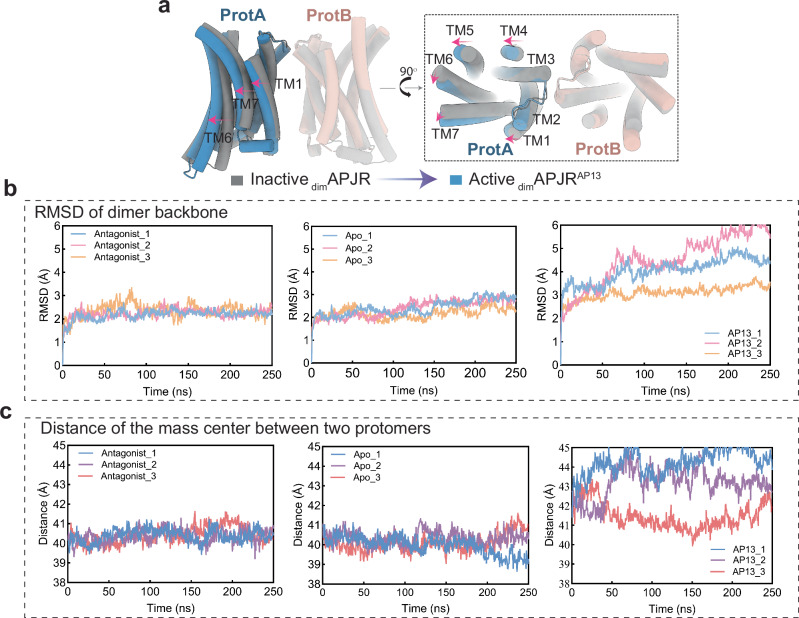
Fig. 4. Dimerization-regulated APJR activation features, ligand-dependent ProtB conformation and dimer stability analysis.
a Structural transitions from inactive-state APJR dimer (JN241-bound symmetric dimer) to active-state APJR dimer (agonist-bound asymmetric dimer) upon activation induced by apelin-13. Superposition of dimAPJRJN241 (inactive dimers) (gray) and dimAPJRAP13–Gi (active dimers) (ProtA: blue, ProtB: pink) on ProtB. Gi protein was omitted. The transitional movement related to ProtA from inactive to active states is indicated with red arrows. b RMSD of APJR dimer backbone in three different states (apo, inactive and apelin-13-bound) in 250 ns MD simulations repeated in triplicates. c Distance of the mass center between two protomers in three different states (apo, inactive and apelin-13 bound) in 250 ns MD simulations repeated in triplicates.