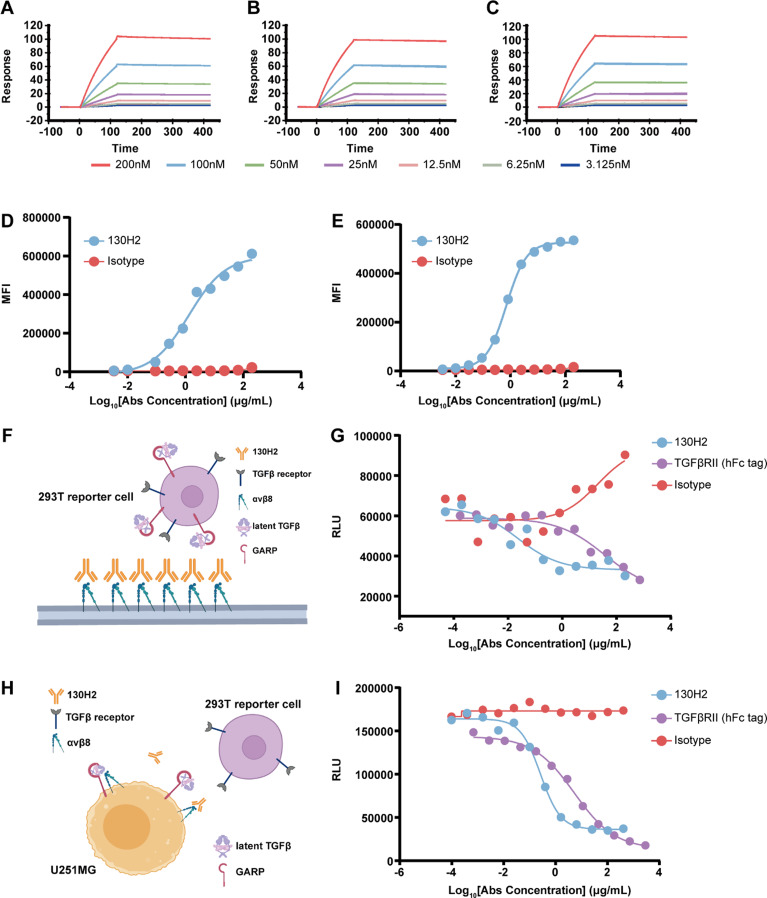
Fig. 2.
In vitro characterization of the anti-αvβ8 antibody 130H2. (A-C) 130H2 demonstrates robust binding capacity to serially diluted (concentration ranging from 200 to 3.125 nM) human αvβ8 protein (A), cynomolgus αvβ8 protein (B), and mouse αvβ8 protein (C) as determined by Biacore system. (D-E) Flow cytometry-based binding curves of threefold diluted 130H2 starting from 200 nM to native U251MG cells (D) and OVCAR3 cells (E) detected by APC labeled secondary antibody. The curves were fitted using sigmoidal four parameter logistic (4PL) model. (F) Schematic illustration of the protein-cell interaction system used in Fig. 2G. The TGF-β luciferase reporter HEK293T cell line, co-transfected with GARP, latent TGF-β1, and TGF-βRII, was used as effector cells. (G) The blocking activity of 130H2 was evaluated by reporter-based assay. Concentration dependent inhibition curves of 130H2 compared with TGFβRII trap were generated by incubating them with αvβ8 and HEK293T/GARP/latent-TGFβ1/TGFβRII-luciferase cells for 5 h and detected by luciferase substrate. (H) Schematic illustration of the cell-cell interaction system used in Fig. 2I. U251MG cells, which natively express both integrin αvβ8 and latent TGF-β1, were used as target cells. The TGF-β luciferase reporter HEK293T cell line, co-transfected with GARP, latent TGF-β1, and TGF-βRII, was used as effector cells. (I) The blocking activity of 130H2 was determined by U251MG-effector cell interaction system. Human HEK293T/GARP/latent-TGFβ1/TGFβRII-luciferase cells were incubated with U251MG cells (E: T = 2: 1) for 5 h in the presence of serial dilutions of 130H2 or TGFβRII trap and the blocking reaction was detected by measuring luminescence units