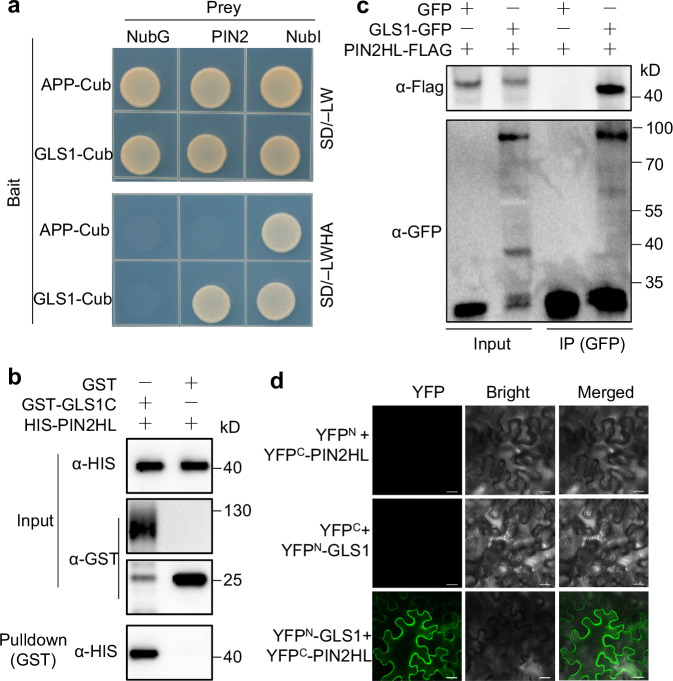
Fig. 4. OsGLS1 physically interacts with OsPIN2.
a Split-ubiquitin yeast two-hybrid analysis. Cub, C-terminal half of ubiquitin (the bait APP-Cub interacts with NubI but not with NubG, which served as positive and negative controls, respectively); NubI and NubG, wild-type and mutated N-terminal fragments of ubiquitin, respectively. Yeast cells were grown on synthetic defined (SD) medium lacking leucine and tryptophan (SD/–LW) or lacking leucine, tryptophan, histidine and adenine (SD/–LWHA). b Pull-down assay. Recombinant GST-GLS1C, OsGLS1C (aa 40–690) fused to a GST tag. HIS-PIN2HL, OsPIN2HL (aa 161–459) fused to a HIS tag. After co-incubating GST-GLS1C with HIS-PIN2HL, proteins were pulled down with glutathione-Superflow resin and detected using anti-HIS antibody. c Co-immunoprecipitation (Co-IP) assays. 35S:OsPIN2HL-FLAG was co-transfected with 35S:OsGLS1-GFP or 35S:GFP in rice protoplasts. Equal amounts of total proteins were immunoprecipitated with anti-GFP antibody–conjugated beads and detected using anti-GFP and anti-Flag antibodies. d BiFC assays. OsGLS1 and OsPIN2HL were fused to the C-terminal (YFPC) and N-terminal (YFPN) halves of YFP, respectively. Combinations of constructs encoding YFPN or YFPC fused to the corresponding OsGLS1 and OsPIN2HL were used as negative controls. Fluorescence was observed using a confocal microscope. Scale bars, 20 μm.