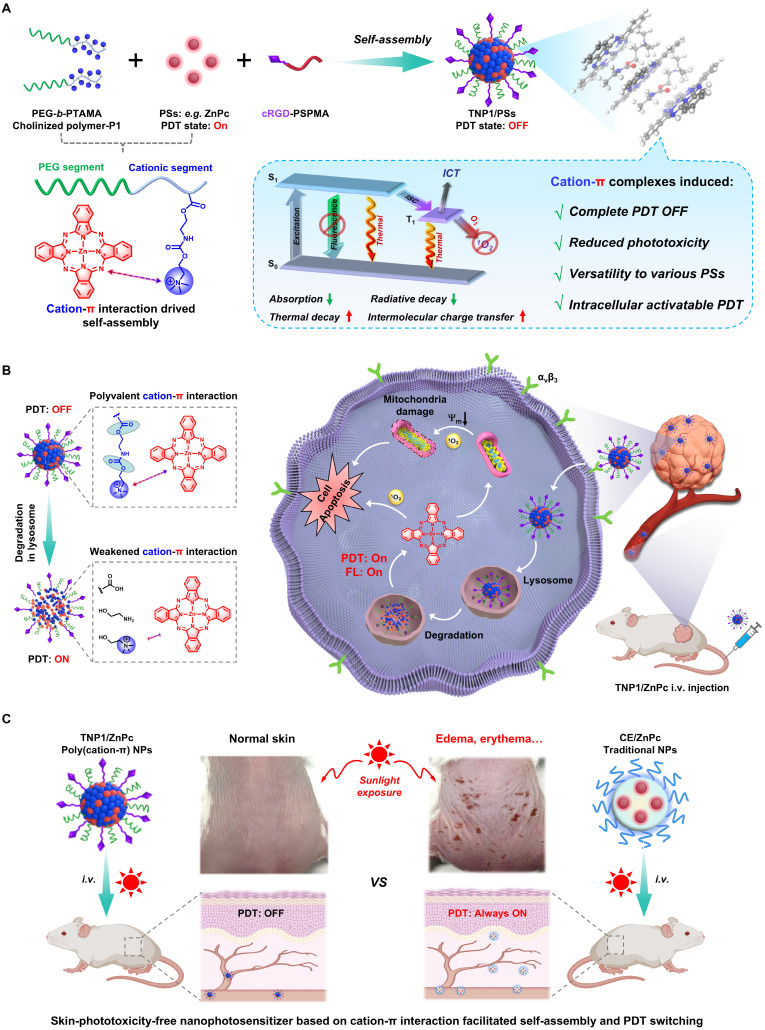
Figure 1.
Schematic illustration of the general strategy towards the construction of activity-switchable nanophotosensitizer for skin-phototoxicity-free and tumor-targeted photodynamic therapy (PDT). (A) The self-assembly of tumor-targeting photosensitizer (PS) called TNP1/PS was driven by cation-π interactions. This interaction occurs between cationic species within cholinized polymer (P1) and aromatic rings within PS. Unexpected, the formation of cation-π complexes was found to induce a complete OFF of the PDT activity of TNP1/PS. This deactivation was discovered to occur through a multi-pathway mechanism, including reduced absorption and radiative decay, while enhanced thermal decay and intermolecular charge transfer. (B) Schematic illustration of the spontaneous recovery of PDT activity of TNP1/PS (e.g. TNP1/ZnPc) in tumor cells via lysosome-mediated degradation, enabling tumor-targeted photodynamic therapy following intravenous administration (i.v.). (C) The skin-phototoxicity-free of nanophotosensitizer in the form of poly(cation-π) NPs (e.g. TNP1/ZnPc), compared to the significant skin-phototoxicity of traditional NPs (e.g. CE/ZnPc formulated by Cremphor EL).