Abstract
Objectives
The objective of this study was to assess the clinical, hemodynamic characteristics and immediate outcomes of Percutaneous Balloon Mitral Valvotomy (PBMV) in low gradient severe rheumatic mitral stenosis (LGMS) with normal cardiac index.
Background
The optimal management of LGMS remains incompletely understood.
Methods
We examined 200 consecutive patients with severe rheumatic mitral stenosis (MS) who underwent PBMV between January 2014 and March 2020.
Results
Of the 149 patients (who satisfied inclusion criteria), 51 (34.2 %) had LGMS. The mean diastolic pressure gradient (DPG) was 8.70 ± 1.34 mm of Hg in LGMS as compared to 16.2 ± 4.3 mm of Hg in HGMS (p < 0.001). Patients of LGMS were older (39.5 ± 9.7 vs.34.9 ± 11.0 years, p = 0.012), had lower baseline heart rate (76.8 ± 9.5 vs 81.9 ± 12.5, p = 0.010), higher Mitral valve area (MVA) (1.16 ± 0.19 vs 0.99 ± 0.21 cm2,p < 0.001),higher Wilkins score (5.8 ± 1.7 vs 4.9 ± 1.5, p = 0.002) and elevated left ventricular end diastolic pressure (LVEDP) (9.2 ± 2.8 vs 5.8 ± 1.2 mm of Hg,p=<0.001) but lower Pulmonary artery systolic pressure (PASP) (53.1 ± 14.5 vs 62.6 ± 17.8 mm of Hg, p = 0.001) and left atrial (LA) pressure (18.0 ± 3.1vs 22.0 ± 4.4 mm of Hg,p=<0.001). Although, the procedural success rate of PBMV was comparable between LGMS and HGMS (92.2 % vs 96.9 % p = 0.231) but increment in MVA and fall in DPG were significantly higher in HGMS in comparison to LGMS (p-value<0.05).
Conclusions
Significant MS may have "low" gradients during catheterization and yet be symptomatic, and thus low gradients cannot be alone used as a marker of disease severity. LGMS with normal CI is characterized by unique clinical and hemodynamic features. The immediate outcome of PBMV is comparable to HGMS but the hemodynamic parameters to monitor the success of PBMV are significantly different.
Keywords: Percutaneous balloon mitral valvotomy, Low gradient severe rheumatic mitral stenosis, Cardiac index, High gradient severe rheumatic mitral stenosis, Diastolic pressure gradient
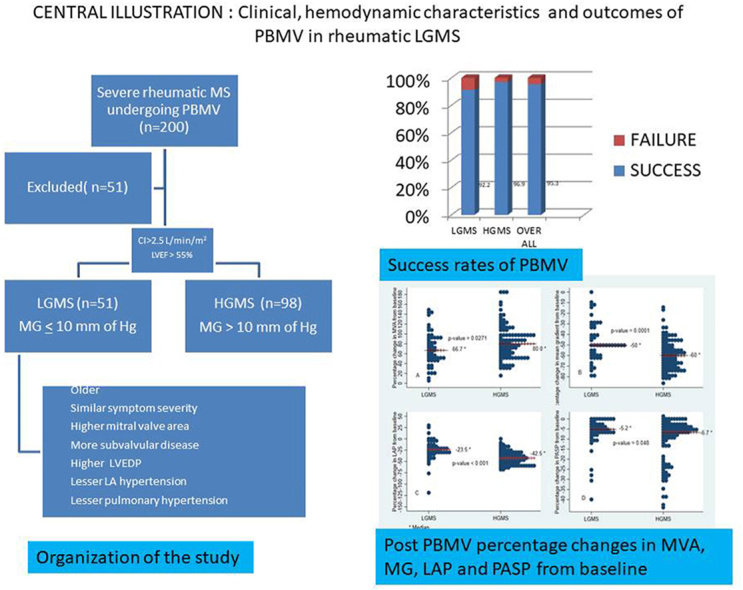
Graphical abstract
Low gradient mitral stenosis (LGMS) with normal CI is characterized by low transmitral DPG (≤10 mm of Hg) despite symptomatic severe mitral stenosis. LGMS patients have symptoms similar in severity to HGMS patients but are usually older, have lower heart rate, higher baseline mitral valve area (MVA),higher left ventricular end diastolic pressure (LVEDP) and more subvalvular involvement with relatively lower pulmonary artery systolic pressure and lesser LA hypertension.
1. Introduction
The major hemodynamic abnormalities of Mitral stenosis (MS) are typically characterized by elevated left atrial pressure (LAP) and high diastolic pressure gradient (DPG) across the mitral valve owing to the obstruction of the left ventricular inflow.1, 2, 3 According to the American Heart Association (AHA) and the American College of Cardiology (ACC) recent guidelines, severe MS is defined as a mitral valve area (MVA) ≤1.5 cm2with Pressure half time (PHT) ≥ 150 msec and is usually associated with high DPG4 and generally MVA ≤ 1 cm2 corresponds to DPG of more than 10 mm of Hg. However, it has been reported that there is a distinct group of patients of severe MS who have initial low DPG (≤10 mm of Hg) at presentation with normal cardiac output and preserved left ventricular ejection fraction (LVEF).5 Although, the DPG across mitral valve is primarily flow dependent, but it can also be influenced by various other parameters such as left ventricular ejection fraction, heart rate, stroke volume, architectural geometry and extent of subvalvular apparatus involvement.3,6, 7, 8, 9 Presently, the precise pathophysiology of low pressure gradient in such patients is not clearly understood but they can be divided in two broad hemodynamic subgroups based upon cardiac index: low-flow/low-gradient (LF/LG) and normal-flow/low-gradient(NF/LG) severe mitral stenosis.10 In patients with LF/LG severe MS, the initial low DPG are attributed to lowerLVEF, low cardiac index (CI), high arterial afterload and reduced ventricular performance due to ventricular –vascular uncoupling,10; the pathophysiology in patients of NF/LG is similar but less pronounced with normal cardiac index. Percutaneous Balloon Mitral valvotomy (PBMV) and its modifications are established and less invasive alternative techniquesto mitral valve replacement(MVR)for symptomatic severe MS with suitable valve morphology.4,11, 12, 13, 14, 15 The clinical outcomes of PBMV in a cohort of severe MS with low DPG remains elusive, but a few studies have reported suboptimal outcomes with both PBMV and MVR in this subgroup of patients of severe MS.10,16,17 The primary objective of this study was to assess the clinical and hemodynamic characteristics of LGMS with normal cardiac index and to evaluate theimmediate outcomes of PBMV in such patients.
2. Methods
2.1. Study population
The study was approved by the institute review board and an independent institutional ethical committee. The medical records of 200consecutive patients who underwent PBMV between January 2014 and March 2020 were analysed retrospectively. These included detailed demography, clinical data, echocardiographic parameters and peri-procedural hemodynamic measurements at baseline and immediately after completion of the procedure. All patients who had severe MS (MVA <1.5 cm2) with normal LVEF, normal CI, and who had undergone PBMV were included for the purpose of study. The authors excluded the patients with (a) coronary artery disease (b) systemic arterial hypertension (c) previous exposure to high –dose mediastinal irradiation or cardiotoxic therapy (d) Significant multivalvular involvement (e) Cardiac index <2.5 L/min/m2.The clinical characteristics, hemodynamic data and outcome of PBMV in patients with low initial DPG were compared to that of patients with high initial DPG.
2.2. Pre-operative evaluation
Comprehensive 2-Dimensional transthoracic Doppler echocardiography was performed to assess the severity of MS, valve morphology, subvalvular apparatus, left ventricular function, mitral regurgitation and to rule out any co-existing structural heart disease. Wilkins score was calculated to predict the success rate of PBMV.18 The severity of MS was assessed by using planimetric MVA method in parasternal short axis view and diastolic PHT. PreproceduralTransesophageal echocardiography (TEE) was performed in all patients to exclude LA thrombus, extensive commissural calcification and significant mitral regurgitation (MR).Patients were considered to have low gradient (LGMS) if their mean transmitral gradient from transthoracic echocardiography was ≤10 mm of Hg. Since it was a retrospective study we did not have systemic data on volume status of the patients and no provocative tests were done to evaluate their effect on MDG.
2.3. PBMV technique and hemodynamic evaluation
All patients underwent PBMV by antegrade trans-septal technique with Inoue balloon catheter via right femoral vein under local anaesthesia without sedation.19 Prophylactic antibiotic was given before operation and heparin was administered only after dilatation of punctured septum. Septal puncture was done by using Brockenbrough needle and Mullins sheath, and the appropriate size of the balloon was selected based on the height of patient. Left and right heart hemodynamic data were measured immediately before and after PBMV. Cardiac output and cardiac index were calculated by using Fick's formula. The immediate outcome of the procedure was assessed invasively by measuring LA pressure as well as non-invasively by 2D echocardiography to calculate MVA and to identify complications like pericardial effusion, significant MR or worsening of pre-existing MR. The procedure was considered successful if MVA increased by 50 % from baseline or absolute MVA was >1.5 cm2 without any major complications.20 The major complications were defined as death, cardiac tamponade and occurrence of moderate to severe MR.12 These percentage change in mean gradient, MVA, LA pressure and PASP were defined as [{(Post-Pre)/Pre}∗100].
2.4. Statistical analysis
Normality test for continuous variables were done using Shapiro–Wilk test. Categorical datawere presented as frequency (percentage), and continuous normally distributed and non-normally distributed variables were reported as means ± standard deviations (SD) and median (minimum–maximum), respectively. To test the association between two categorical variables, Pearson chi-square/Fisher's exact test were used. Normally and non-normally distributed continuous variables between two independent groups were compared using Student's t-test and Wilcoxon rank-sum test, respectively. All the p-values less than 0.05 were taken as statistically significant. All the analysis was performed using Stata 13.0 software.
3. Results
3.1. Baseline demographic and clinical data
Between January 2014 and March 2020, a total of 149 patients, who underwent PBMV, and satisfied the inclusion criteria were analysed. Of the 149 patients, 51 (34.2 %) had Low DPG severe Mitral stenosis (LGMS), while 98 (65.8 %) had high DPG severe mitral stenosis (HGMS) Baseline demographic and clinical data of the LGMS compared with the HGMS are summarized in Table 1.
Table 1.
Demographic and clinical data.
| Variables | LGMS (n = 51) | HGMS (n = 98) | p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 39.5 ± 9.7 | 34.9 ± 11.0 | 0.0128 |
| Gender | |||
| Male | 18 (35.3 %) | 26 (26.5 %) | 0.266 |
| Female | 33 (64.7 %) | 72 (73.5 %) | |
| BSA (Body surface area) [ per metre2] | 1.45 ± 0.17 | 1.41 ± 0.17 | 0.215 |
| BMI (Body mass index) [kg/m2] | 20.4 ± 3.4 | 20.3 ± 3.5 | 0.836 |
| Hb (hemoglobin) [g/dl] | 13.1 ± 1.8 | 12.7 ± 1.7 | 0.151 |
| Medication | |||
| Beta-blocker | 36 (70.6 %) | 85 (86.7 %) | 0.017 |
| Digoxin | 2 (3.9 %) | 6 (6.1 %) | 0.716 |
| Diltiazem | 12 (23.5 %) | 20 (20.4 %) | 0.660 |
| Symptoms | |||
| NYHA | |||
| 1 | 0 | 0 | 0.785 |
| 2 | 31 (62 %) | 65 (67 %) | |
| 3 | 16 (32 %) | 28 (28.9 %) | |
| 4 | 3 (6 %) | 4 (4.1 %) | |
| Rhythm | |||
| Atrial fibrillation | 19 (37.3 %) | 36 (36.7 %) | 0.481 |
| Atrial flutter | 1 (1.9 %) | 0 | |
| Normal sinus rhythm | 31 (60.8 %) | 62 (63.3 %) | |
HGMS- high gradient mitral stenosis; LGMS- low gradient mitral stenosis, NYHA- New York Heart Association.
When compared to HGMS, patients with LGMS were older (39.5 ± 9.7 vs.34.9 ± 11.0 years p = 0.0128),had lower baseline heart rate (76.8 ± 9.5 vs 81.9 ± 12.5, p = 0.0103)and lesser usage of Beta-blockers(70.6 % Vs 86.7 %, p = 0.017).There was no significant difference between the two groups pertaining to gender, body surface area (BSA), body mass index (BMI),haemoglobin(Hb), prevalence of atrial fibrillation (AF) and usage of other drugs like digoxin and diltiazem. Both the groups had similar severity of symptoms and majority of patients in both groups had baseline New York Heart Association (NYHA) functional class II symptoms and only 6 % and 4.1 % had severe symptoms (NYHA class IV) in LGMS and HGMS respectively.
3.2. Pre-PBMV echocardiographic and hemodynamic data
As summarized in Table 2, all subjects in both the groups had normal LVEF and normal CI. The mean of echocardiography derived DPG was 8.70 ± 1.34 mm of Hg in LGMS as compared to 16.2 ± 4.3 mm of Hg in HGMS (p < 0.001).Patients with LGMS had higher value of pre-PBMV MVA (1.16 ± 0.19vs0.99 ± 0.21 cm2, p < 0.001) and Wilkins score (5.8 ± 1.7 vs 4.9 ± 1.5, p = 0.002), but lower PASP (53.1 ± 14.5 vs 62.6 ± 17.8 mm of Hg, p = 0.001)0.62.7 % of patients from Low gradient group and 37.8 % of patients from high gradient group had MVA between 1.0 and 1.5 cm2. LGMS patient's subvalvular score was significantly higher. However, differences in other echocardiographic parameters such as LA size and MR were not statistically significant between the two groups. Invasive hemodynamic data related to Stroke volume, CI were comparable between the two groups except for the higher LVEDP (9.2 ± 2.8 vs 5.8 ± 1.2 mm of Hg, p=<0.001) and lower LA pressures (18.0 ± 3.1vs 22.0 ± 4.4 mm of Hg, p=<0.001) in LGMS group.
Table 2.
Pre PBMV echocardiographic and hemodynamic data.
| Variables | LGMS(n = 51) | HGMS (n = 98) | p-value |
|---|---|---|---|
| Echocardiography | |||
| Mitral valve area [cm2] | 1.16 ± 0.19 | 0.99 ± 0.21 | <0.001 |
| Wilkins score | 5.8 ± 1.7 | 4.9 ± 1.5 | 0.0020 |
| Subvalvular score | |||
| 1 | 17 (33.3 %) | 85 (86.7 %) | <0.001 |
| 2 | 15 (29.4 %) | 13 (13.3 %) | |
| 3 | 19 (37.3 %) | 0 | |
| 4 | 0 | 0 | |
| Pulmonary artery systolic pressure [mmHg] | 53.1 ± 14.5 | 62.6 ± 17.8 | 0.0013 |
| Mean diastolic gradient [mmHg] | 8.70 ± 1.34 | 16.2 ± 4.3 | <0.001 |
| Left atrial size (mm) | 4.45 ± 0.50 | 4.42 ± 0.50 | 0.722 |
| Mitral regurgitation | |||
| Nil | 46 (90.2 %) | 80 (81.6 %) | 0.170 |
| Mild | 5 (9.8 %) | 18 (18.4 %) | |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 | |
| Left Ventricular ejection fraction | |||
| <55 % | 0 | 0 | |
| ≥55 % | 51 (100 %) | 98 (100 %) | |
| Catheterization | |||
| Heart rate(/min) | 76.8 ± 9.5 | 81.9 ± 12.5 | 0.0103 |
| Cardiac output [L/min] | 4.05 ± 0.59 | 4.08 ± 0.55 | 0.752 |
| Cardiac Index) [L/min/m2] | 2.7 ± 0.30 | 2.8 ± 0.36 | 0.125 |
| Stroke volume) [ml/beat] | 53.5 ± 10.5 | 51.3 ± 11.3 | 0.229 |
| Left atrial pressure [mmHg] | 18.0 ± 3.1 | 22.0 ± 4.4 | <0.001 |
| Left ventricular end diastolic pressure (LVED) [mmHg] | 9.2 ± 2.8 | 5.8 ± 1.2 | <0.001 |
HGMS- high gradient mitral stenosis; LGMS- low gradient mitral stenosis, NYHA- New York Heart Association.
3.3. Outcomes of PBMV
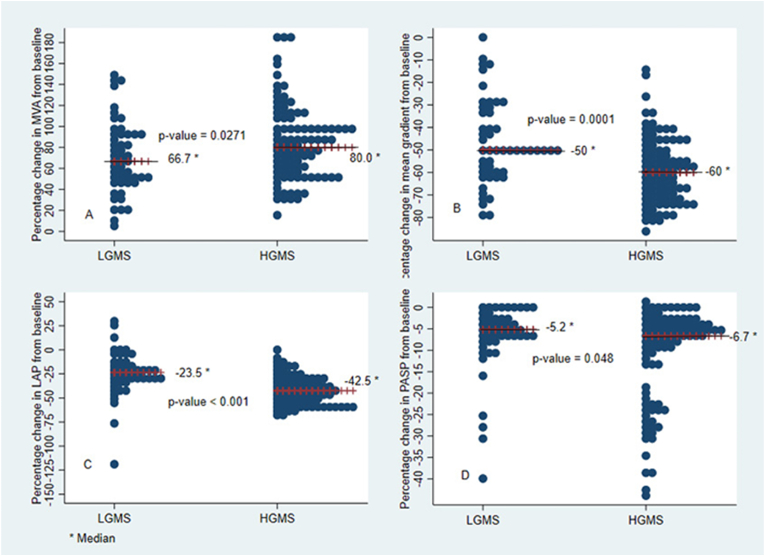
The overall procedural success rate was 95.3 % (n = 142/149) and itwas comparable between the two groups i.e.92.2 % (47/51) and 96.9 % (95/98) for LGMS and HGMS respectively (p = 0.231).Both groups responded well to PBMV with an improvement in MVA (Δ MVA 0.7 ± 0.3vs0.8 ± 0.2 cm2, p = 0.812).The change (fall) in transmitral DPG and LA pressure and PASP immediately after PBMV were significantly more among patients with HGMS as compared to those with LGMS. Major Complications like cardiac tamponade and death were not observed in both groups however, one patient in the LGMS subset developed severe MR following PBMV. The comparison of PBMV outcomes related to clinical, echocardiographic and hemodynamic data between the two groups are summarized in Table 3.Fig. 1 depicts the immediate outcomes of PBMV between the two groups, pertaining to percentage change in mean gradient, MVA, LA pressure and PASP in respective directions. The median value of percentage change in MVA from base line was significantly higher in HGMS as compared to LGMS (66.7 %vs 80 %, p-value = 0.0271). Percentage change in mean gradient, LA pressure and PASP were also found to be significantly higher in HGMS as compared to LGMS (p-value<0.05). On further analysis, none of the variables including mean gradient were found associated with the procedural success rate at 5 % level of significance (Suppl. Table 1). Also, we explored multivariable analysis, for predictors of success rate, including only those factors which turned out significant in univariate analysis at 20 % level of significance, but none of the factors came significant at 5 % level of significance in multivariable analysis.
Table 3.
Immediate outcomes of PBMV.
| Variables | LGMS (n = 51) | HGMS (n = 98) | p-value |
|---|---|---|---|
| Procedural Success [No. (%)] | 47 (92.2) | 95 (96.9) | 0.231 |
| Post-valvuloplasty | |||
| Mitral valve area[cm2] | 1.93 ± 0.29 | 1.89 ± 1.16 | 0.805 |
| Mean gradient [mmHg] | 4.58 ± 1.63 | 6.33 ± 2.14 | <0.001 |
| Pulmonary artery systolic pressure [mmHg] | 53.2 ± 27.6 | 54.6 ± 13.0 | 0.685 |
| Mitral regurgitation | |||
| Mild | 4 (7.8 %) | 5 (5.1 %) | 0.019 |
| Moderate | 1 (1.9 %) | 15 (15.3 %) | |
| Severe | 1 (1.9 %) | 0 | |
| Left Atrial pressure (mmHg) | 13.±4.8 | 12.4 ± 2.9 | 0.032 |
| Change | |||
| Δ MVA (cm2) | 0.7 ± 0.3 | 0.8 ± 0.2 | 0.812 |
| ΔMG (mmHg) | 5(0,8) | 9(2,22) | <0.001 |
| Δ LA pressure (mmHg) | 4.2 ± 3.7 | 9.6 ± 4.5 | <0.001 |
| Δ PASP (mmHg) | 3(0,26) | 3.5 (-1,38) | 0.0144 |
[Δ (delta) represents change in absolute value before and after Valvotomy].
MVA- Mitral valve area, MG = Mean gradient, LA- Left atrium, PASP= Pulmonary artery systolic pressure.
Fig. 1.
Post PBMV percentage changes in MVA, MG, LAP and PASP from baseline. Median value of percentage change is indicated with solid line in the graph. Fig. 1 (A): Depicts the median value of percentage change (increase) in MVA from baseline in both groups. HGMS patients had median increment of 80 % of valve area from baseline as compared to median of 66.7 % increment in LGMS (p = 0.0271). Fig. 1(B): Depicts the median value of percentage change (decrease) in MG from baseline in both groups. HGMS patients had median decrement of 60 % of MG from baseline as compared to median of 50 % decrement in LGMS (p = 0.0001). Fig. 1(C): Depicts the median value of percentage change (decrease) in LAP from baseline in both groups. HGMS patients had median decrement of 42.5 % of LAP from baseline as compared to median of 23.5 % decrement in LGMS (p=<0.001). Fig. 1(D): Depicts the median value of percentage change (decrease) in PASP from baseline in both groups. HGMS patients had median decrement of 6.7 % of PASP from baseline as compared to median of 5.2 % decrement in LGMS (p = 0.048). MVA- Mitral valve area, MG- Mean gradient, LAP- Left atrial pressure, PASP- Pulmonary artery systolic pressure.
What is already known?.
There is a unique subset of severe mitral stenosis (MS) patients who have normal ventricular function and preserved cardiac index and still have low transmitral gradients (LGMS).
They are characterized by Older age at presentation, Similar symptom severity to high gradient MS(HGMS), higher mitral valve area, more subvalvular disease, higher left ventricular filling pressures, lesser left atrial hypertension and lesser pulmonary hypertension.
What this study adds?.
We found that the success rate percutaneous valvuloplasty is similar in LGMS and HGMS. However,the despite achieving final area of more than 1.5 cm2, more than half of patients do not achieve >50 % fall in mean DPG.
4. Discussion
Ours is the first study from India that characterizes a unique subset of rheumatic severe MS patients, who have low transmitral diastolic gradient with normal cardiac index at presentation and who underwent PBMV. The authors have studied the frequency, hemodynamics, optimal monitoring strategy of PBMV and the immediate outcomes of PBMV in LGMS patients.
Previous studies have found that up to one third of patients of rheumatic MS have resting LVEF <50 %21,22 and usually with advanced LV dysfunction and low CI they have low DPG.3,6 Additionally, presence of RV dysfunction can lead to reduced Cardiac index and subsequent low DPG. However, there is a subset of patients who have normal ventricular function and preserved cardiac index and still have low transmitral gradients. This subset is important not only because they constitute sizable number of real world patients who undergo PBMV but they are thought to represent pseudo severe form of MS, having lesser response to valvuloplasty and less long term benefits as well.6,10,16
The aetiology of MS in our study was rheumatic and 34.2 % (n = 51/149) represented a group of LGMS. Similar to previous studies, most patients were female (70.5 %) and gender difference between LGMS Vs HGMS group was insignificant. Due to paucity of data, the exact prevalence of LGMS and its hemodynamic subsets (NF/LG and LF/LG) is not clear and the reported prevalence varies from 12 to 55 %.6,10,16 However, these estimates may not represent the true burden of the disease owing to selection bias. In a study of outcomes of PBMV in patients with severe MS and low DGP by Tugrmen et al,16 20 % of the patients had low DPG at presentation out of which 33 % had normal LVEF, indirectly implicating that the proportion of NF/LG was around 6 %. Similarly, El Sabbagh10 et al reported overall 54 % prevalence of LGMS in their study population of 101 consecutive patients with severe MS, out of which 11 % patients were LF/LG and 44 % had NF/LG.
In our study LGMS was found to be associated with older age, lower baseline heart rate, higher Wilkins and Subvalvular score on echocardiography and lower PASP. Similar to our observations Rayburn and Fortuin et al also observed extensive subvalvular disease and lower mean pulmonary artery pressures in such patients.6 These findings signify that poor compliance of left ventricle, due to extensive involvement of subvalvular apparatus and altered geometry could be one of the reasons for causing low mean gradient across mitral valve. These patients are also characterized by higher effective arterial elastance, reduced ventricular capacitance and increase chambers stiffness leading to after load mismatch and ventricular vascular uncoupling rather than intrinsic contractile dysfunction,10 similar to what has been described in patients with paradoxical low flow-low gradient sub-group of aortic stenosis.23,24 We observed that LGMS group had higher baseline MVA, elevated LVEDP and lesser LA pressure with preserved ejection fraction, probably representing a lesser degree of rheumatic MS on one hand; While,the presence of symptoms similar in severity to HGMS support that the symptoms in LGMS patients are primarily driven by prominent subvalvalvular disease,arterial stiffness, ventricular–vascular uncoupling,AF and decreased LV compliance rather than true intrinsic severe MS.10 Many of the previous studies have identified myocardial abnormalities and increased afterload in rheumatic MS.7,25, 26, 27, 28, 29, 30, 31, 32
All subjects responded well to valvuloplasty as per the definition and the procedural success rate was comparable between the two groups (92.2 % vs 96.9 %, p = 0.231).20 Previous studies have reported that after successful PBMV the DPG falls and more than 50 % reduction from baseline correlates with final MVA ≥1.5 cm2.33,34 However we found that this may not be applicable equally to LGMS patients. Despite the achievement of final MVA of MVA ≥1.5 cm2 less than half of patients in LGMS subset achieved 50 % or more reduction in the mean DPG (Fig. 1 A). Also as compared to HGMS the mean fall in LAP was significantly lesser (4.2 ± 3.7 vs 9.6 ± 4.5 mm of Hg, p=<0.001). This finding was similar to a previous study where both LGMS and HGMS responded well to valvuloplasty, but lesser reduction in MG and LA pressure among patients with LGMS10. One study demonstrated that while 82 % of HGMS had reduction in MG ≥ 50 % after PBMV, only 11 % of LGMS had 50 % reduction of MG.16 The baseline MG is already lower among LGMS and majority do not achieve more than 50 % reduction in DPG, therefore it is prudent to consider a change in MVA as success criteria after PBMV, rather than fall in MG. Transthoracic echocardiography is a very useful and complementary method of hemodynamic assessment as well as for reference measurement of MVA during PBMV including bi-commissural splitting.35 The lesser degree of changes in these parameters also suggest that causes other than valvualar obstruction are also accountable for LGMS.
Previous studies have shown that the in the long term the presence of initial low gradient in patients with severe MS, who underwent either PBMV or MVR, was associated with lesser symptomatic benefit.6,10,16 However as follow up data was not available in our study, long term outcomes of valvuloplasty cannot be commented.
Identifying the subset of LGMS and further categorizing them into two distinct subgroup of NF/LG and LF/LG at the initial evaluation will be prudent for many reasons. First of all, LGMS constitutes a significant proportion of real world MS patients and once labelled so, their further management will require more intense efforts in their subsequent evaluation, prognostication, and future treatment and follow up as well.Secondly, this population of patients are candidates for more intense initial scrutiny by utilizing newer measures such as strain rate imaging, tissue Doppler imaging etc. for assessment of LV compliance, LVEDP, subclinical LV systolic dysfunction in order to overcome the limitations of 2D- Echo based evaluation and sub categorise them as LF/LG or NF/LG severe MS. Thirdly, while discussing the overall pre-treatment plan, during pre-procedural counselling the patients of LGMS might be appropriately prognosticated about the expected outcome considering relatively poor intermediate and long term symptomatic improvement observed in clinical studies. Fourthly, besides PBMV; depending upon the cause of low transmitral gradient (either due to noncompliant LV, systolic dysfunction or increased arterial after load) and no or less symptomatic relief; the subsequent treatment of LGMS patients might also include standard therapies for heart failure with preserved or reduced ejection fraction. Lastly, during subsequent follow up of patients of NF/LG MS who develop restenosis, considering the lesser degree of decrease in LA pressure, mean gradient and immediate gain in MVA, mitral valve repair/replacement might be offered as a reasonable alternative.
4.1. Limitations
There is a selection bias in our study population since we included patients who underwent PBMV and other limitations of retrospective studies are also applicable. This is a retrospective study and we could not systematically evaluate volume status of the patients which can significantly contribute to the MDG at baseline. Another major limitation is that no provocative tests such as exercise testing were performed to evaluate its effect on gradients and PA pressures. Our study provides a impetus to evaluate LGMS cases systematically.
The echocardiographic and catheterization data measurements were not done simultaneously and most importantly follow up data are not available with us to determine the intermediate and long term outcomes of PBMV in LGMS patients.Another limitation of our study is the missing data regarding important hemodynamic parameters like systemic vascular resistance and pulmonary vascular resistance which could have given further insights to our findings. It would be interesting to evaluate the relation between exercise and DPG in such patients in future studies.
5. Conclusion
Significant MS may have "low" gradients during catheterization and yet be symptomatic, and thus low gradients as such cannot be alone used as a marker of disease severity.
Low gradient severe MS with preserved LVEF is a unique entity and commonly encountered among those who undergo PBMV. This subgroup of LGMS have symptoms similar in severity to HGMS but are usually older, have lower baseline heart rates, higher baseline MVA and more subvalvular involvement with lower PASP. The low DPG is either a consequence combination of factors including extensive involvement of subvalvular apparatus,higher effective arterial elastance, reduced ventricular capacitance and increase chambers stiffness leading to after load mismatch and ventricular vascular uncoupling.However volume status of patients must be taken into account (given the fact majority of them are on diuretics) which can significantly affect the MDG. Also, provocative tests such as exercise tests must be done to see the response on MDG and PA pressures. The immediate procedural success rate of PBMV among LGMS is comparable to HGMS. However, more than half of LGMS patients do not achieve ≥50 % fall in mean DPG and thus it is also prudent to consider a change in MVA (planimetry derived) as a success criterion after PBMV.
Data availability statement
The data related to this study are available with the corresponding author on reasonable request. Sharing of the patient data is subject to limitations of informed consent and approval by the Institutional review board.
CRediT author statement
AM, VF, MK, and AM were involved in patient care, drafting and editing of the manuscript. VK was involved in data analysis, drafting and editing of manuscript.
Funding/disclosure
All authors have nothing to disclose, no relationship to industry.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviation:
- ACC
American College of Cardiology
- AHA
American Heart Association
- CI
Cardiac index
- DPG
Diastolic pressure gradient
- HGMS
High gradient mitral stenosis
- LGMS
Low gradient mitral stenosis
- LAP
Left atrial pressure
- LF/LG
Low-flow/low-gradient
- LVEDP
Left ventricular end diastolic pressure
- LVEF
Left ventricular ejection fraction
- MR
Mitral regurgitation
- MS
Mitral stenosis
- MVA
Mitral valve area
- MVR
Mitral valve replacement
- NF/LG
Normal-flow/low-gradient
- PBMV
Percutaneous Balloon Mitral Valvotomy
- TEE
Transesophageal echocardiography
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2024.11.333.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Braunwald E., Moscovitz H.L., Amram S.S., et al. The hemodynamics of the left side of the heart as studied by simultaneous left atrial, left ventricular, and aortic pressures; particular reference to mitral stenosis. Circulation. 1955;12:69–81. doi: 10.1161/01.cir.12.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Rowe J.C., Bland E.F., Sprague H.B., White P.D. The course of mitral stenosis without surgery: ten- and twenty-year perspectives. Ann Intern Med. 1960;52:741. doi: 10.7326/0003-4819-52-4-741. [DOI] [PubMed] [Google Scholar]
- 3.Hugenholtz P.G., Ryan T.J., Stein S.W., Abelmann W.H. The spectrum of pure mitral stenosis. Hemodynamic studies in relation to clinical disability. Am J Cardiol. 1962;10:773–784. doi: 10.1016/0002-9149(62)90171-6. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura R.A., Otto C.M., Bonow R.O., et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252. doi: 10.1016/j.jacc.2017.03.011. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd G., Badiani S., Costa M., Armado K., Bhattacharyya S. Mitral stenosis in 2019: changing approaches for changing times. Expert Rev Cardiovasc Ther. 2019 Jul;17(7):473–477. doi: 10.1080/14779072.2019.1632190. [DOI] [PubMed] [Google Scholar]
- 6.Rayburn B.K., Fortuin N.J. Severely symptomatic mitral stenosis with a low gradient: a case for low-technology medicine. Am Heart J. 1996;132(3):628–632. doi: 10.1016/s0002-8703(96)90248-3. [DOI] [PubMed] [Google Scholar]
- 7.Holzer J.A., Karliner J.S., O'Rourke R.A., Peterson K.L. Quantitative angiographic analysis of the left ventricle in patients with isolated rheumatic mitral stenosis. Br Heart J. 1973;35(5):497–502. doi: 10.1136/hrt.35.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgartner H., Hung J., Bermejo J., et al. American Society of Echocardiography European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009 Jan;22(1):1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Mohan J.C., Patel A.R., Passey R., et al. Is the mitral valve area flow-dependent in mitral stenosis? A dobutamine stress echocardiographic study. J Am Coll Cardiol. 2002;40:1809–1815. doi: 10.1016/s0735-1097(02)02487-7. [DOI] [PubMed] [Google Scholar]
- 10.El Sabbagh A., Reddy Y.N.V., Barros-Gomes S., et al. Low-gradient severe mitral stenosis: hemodynamic profiles, clinical characteristics, and outcomes. J Am Heart Assoc. 2019 Mar;8(5) doi: 10.1161/jaha.118.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 12.Lock J.E., Kalilullah M., Shrivastava S., et al. Percutaneous catheter commissurotomy in rheumatic mitral stenosis. N Engl J Med. 1985;313:1515–1518. doi: 10.1056/NEJM198512123132405. [DOI] [PubMed] [Google Scholar]
- 13.Nobuyoshi M., Hamasaki N., Kimura T., et al. Indications, complications and short-term clinical outcome of percutaneous transvenous mitral commissurotomy. Circulation. 1989;80:782–792. doi: 10.1161/01.cir.80.4.782. [DOI] [PubMed] [Google Scholar]
- 14.Stefanadis C., Stratos C., Pitsavos C., et al. Retrograde nontransseptal balloon mitral valvuloplasty. Immediate results and long-term follow-up. Circulation. 1992;85:1760–1767. doi: 10.1161/01.cir.85.5.1760. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz C., Zhang H.P., Macaya C., et al. Comparison of Inoue single balloon versus double balloon technique for percutaneous mitral valvotomy. Am Heart J. 1992;123:942–947. doi: 10.1016/0002-8703(92)90700-6. [DOI] [PubMed] [Google Scholar]
- 16.Turgeman Y., Atar S., Suleiman K., Bloch L., Rosenfeld T. Percutaneous balloon mitral valvuloplasty in patients with severe mitral stenosis and low transmitral diastolic pressure gradient. Int J CardiovascIntervent. 2003;5(4):200–205. doi: 10.1080/14628840310016862. [DOI] [PubMed] [Google Scholar]
- 17.Cho I.J., Hong G.R., Lee S.H., et al. Differences in characteristics, left atrial reverse remodeling, and functional outcomes after mitral valve replacement in patients with low-gradient very severe mitral stenosis. J Am Soc Echocardiogr. 2016 Aug;29(8):759–767. doi: 10.1016/j.echo.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Ellis L.B., Harken D.E. BlackH.Aclinical study of 1,000 consecutive cases of mitral stenosis two to nine years after mitral valvuloplasty. Circulation. 1959;19:803–820. doi: 10.1161/01.cir.19.6.803. [DOI] [PubMed] [Google Scholar]
- 19.Croft C.H., Lipscomb K. Modified technique of transseptal left heart catheterization. J Am Coll Cardiol. 1985;5(4):904–910. doi: 10.1016/s0735-1097(85)80431-9. [DOI] [PubMed] [Google Scholar]
- 20.Hogan K., Ramaswamy K., Losordo D.W., et al. Pathology of mitral commissurotomy performed with the Inoue catheter: implications for mechanisms and complications. Cathet Cardiovasc Diagn. 1994;(suppl 2):42–51. [PubMed] [Google Scholar]
- 21.McDonald I.G. Echocardiographic assessment of left ventricular function in mitral valve disease. Circulation. 1976;53:865–871. doi: 10.1161/01.cir.53.5.865. [DOI] [PubMed] [Google Scholar]
- 22.LevinsonGE FrankMJ., UadimiM BraunsteinM. Studies of cardiopulmonary blood volume. Measurement of left ventricular volume by dye dilution. Circulation. 1967;35:1038–1048. doi: 10.1161/01.cir.35.6.1038. [DOI] [PubMed] [Google Scholar]
- 23.Eleid M.F., Nishimura R.A., Sorajja P., Borlaug B.A. Systemic hypertension in low gradient severe aortic stenosis with preserved ejection fraction. Circulation. 2013;128:1349–1353. doi: 10.1161/CIRCULATIONAHA.113.003071. [DOI] [PubMed] [Google Scholar]
- 24.Eleid M.F., Nishimura R.A., Borlaug B.A., Sorajja P. Invasive measures of afterload in low gradient severe aortic stenosis with preserved ejection fraction. Circ Heart Fail. 2013;6:703–710. doi: 10.1161/CIRCHEARTFAILURE.112.000164. [DOI] [PubMed] [Google Scholar]
- 25.Gash A.K., Carabello B.A., Cepin D., Spann J.F. Left ventricular ejectionperformance and systolic muscle function in patients with mitral stenosis. Circulation. 1983;67:148–154. doi: 10.1161/01.cir.67.1.148. [DOI] [PubMed] [Google Scholar]
- 26.Dutrey D.E., Drake E.H. Preprocedural diagnosis of acquired valvulardisease.Evaluation of different diagnostic methods with special emphasis on left heartcatheterization and comparison with surgical findings. Am J Cardiol. 1961;8:319–327. doi: 10.1016/0002-9149(61)90150-3. [DOI] [PubMed] [Google Scholar]
- 27.Bolen J.L., Lopes M.G., Harrison D.C., Alderman E.L. Analysis of left ventricularfunction in response to afterload changes in patients with mitral stenosis. Circulation. 1975;52:894–900. doi: 10.1161/01.cir.52.5.894. [DOI] [PubMed] [Google Scholar]
- 28.Fleming H.A., Wood P. The myocardial factor in mitral valve disease. Br Heart J. 1959;21:117–122. doi: 10.1136/hrt.21.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaasch W.H., Folland E.D. Left ventricular function in rheumatic mitral stenosis. Eur Heart J. 1991;12(suppl B):66–69. doi: 10.1093/eurheartj/12.suppl_b.66. [DOI] [PubMed] [Google Scholar]
- 30.Harvey R.M., Ferrer I., Samet P., et al. Mechanical and myocardial factors in rheumatic heart disease with mitralstenosis. Circulation. 1955;11:531–551. doi: 10.1161/01.cir.11.4.531. [DOI] [PubMed] [Google Scholar]
- 31.Heller S.J., Carleton R.A. Abnormal left ventricular contraction in patientswithmitral stenosis. Circulation. 1970;42:1099–1110. doi: 10.1161/01.cir.42.6.1099. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz L.D., Mullins C.B., Payne R.M., Curry G.C. Left ventricular function in mitralstenosis. Chest. 1973;64:609–614. doi: 10.1378/chest.64.5.609. [DOI] [PubMed] [Google Scholar]
- 33.Hermann H.C. Acute and chronic efficacy of percutaneous transvenous mitral commissurotomy: implication for patient selection. Cathet Cardiovasc Diagn. 1994;2:61–68. [PubMed] [Google Scholar]
- 34.Inoue K., Owaki T., Nakamura T., Katamura F., Myamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:34–402. [PubMed] [Google Scholar]
- 35.Vahanian A., Acar J. In: Textbook of Interventional Cardiology. Topol E.J., editor. WB Saunders; Philadelphia, PA: 1994. Mitral valvuloplasty: the French experience; pp. 1206–1225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data related to this study are available with the corresponding author on reasonable request. Sharing of the patient data is subject to limitations of informed consent and approval by the Institutional review board.