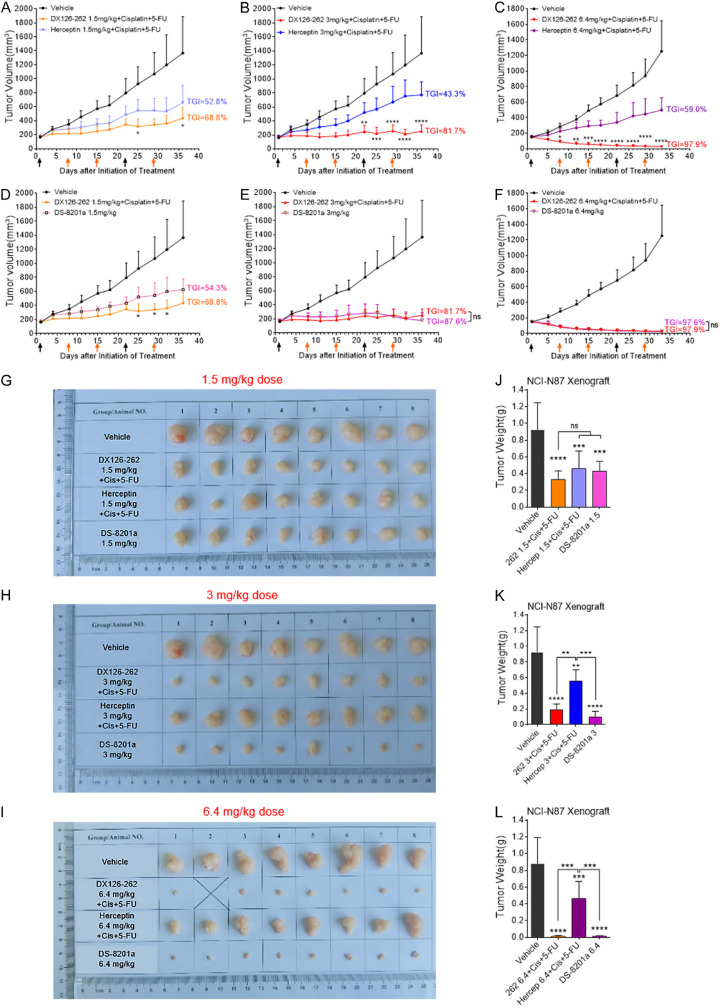
Figure 5.
DX126-262+Cisplatin+5-FU triple therapy showed relatively better in vivo efficacy when compared with Herceptin+Cisplatin+5-FU triple therapy or DS-8201a. (A-C) Antitumor efficacy of DX126-262 combined with Cisplatin and 5-FU were compared with the first-line standard of care of Herceptin triple therapy at the same dose in NCI-N87 xenograft model. The tumor-bearing mice were intravenously administered with three different doses of DX126-262 or Herceptin (1.5 mg/kg dose in A, 3 mg/kg dose in B, 6.4 mg/kg dose in C, day 1, 22), at the same time combined with the same dose of Cisplatin (1 mg/kg, day 1, 22) plus 5-FU (15 mg/kg, day 1, 8, 15, 22, 29). The arrow indicates the date of each intravenous administration. Each point represents the Mean tumor volume and SD (n=8). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, DX126-262+Cisplatin+5-FU versus Herceptin+Cisplatin+5-FU. (D-F) Antitumor efficacy of DX126-262 combined with Cisplatin and 5-FU was compared with the same dose group of DS-8201a in NCI-N87 xenograft model. *P<0.05, DX126-262+Cisplatin+5-FU versus DS-8201a. (G-I) Sizes of exfoliated NCI-N87 xenograft tissues. (J-L) Tumor weights of NCI-N87 xenografts were represented as Mean ± SD. ns: no significance, **P<0.01, ***P<0.001, ****P<0.0001.