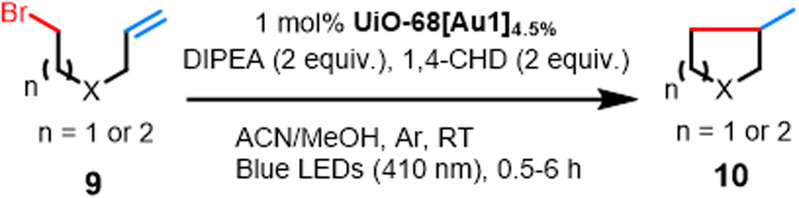
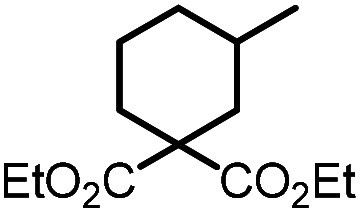
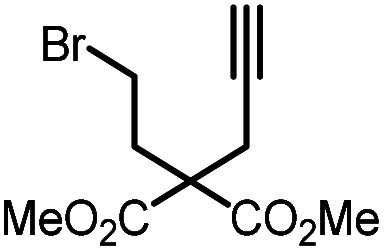
Table 5. Visible-light-promoted reductive cyclization of alkyl halides facilitated by UiO-68[Au1]4.5%a.
| |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Conversion; yield | ||
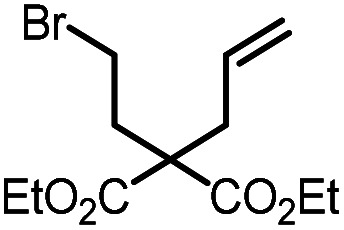
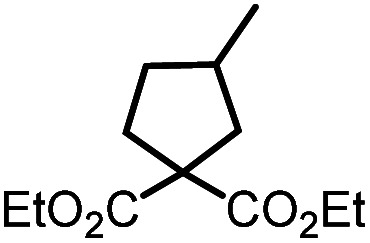
| 1 |
|
9a |
|
10a | 99%; 97% |
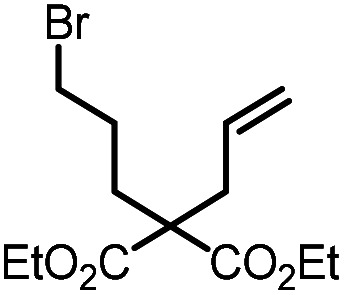
| 2 |
|
9b |
|
10b | 95%; 85% |
| 3 |
|
9c |
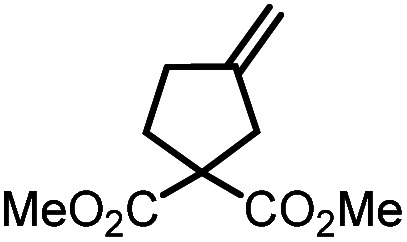
|
10c | 99%; 96% |
| 4 |
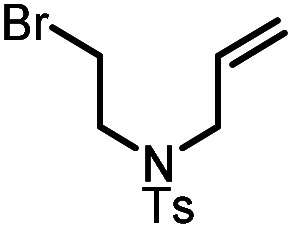
|
9d |
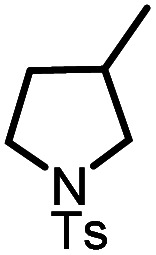
|
10d | 99%; 97% |
| 5 |
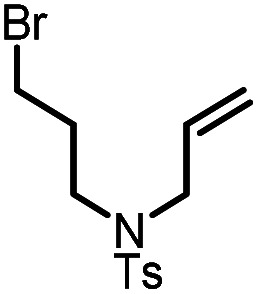
|
9e |
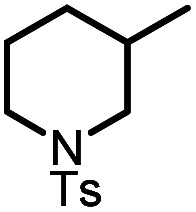
|
10e | 95%; 90% |
| 6 |
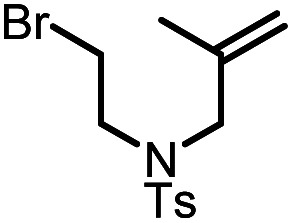
|
9f |
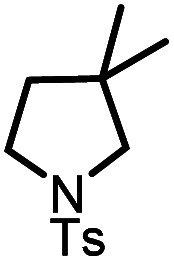
|
10f | 99%; 95% |
Conditions: 9a–9f (0.1 mmol), diisopropylamine (DIPEA) (0.2 mmol), 1,4-cyclohexadiene (1,4-CHD) (0.2 mmol), and UiO-68[Au1]4.5% (5 mg) in a MeCN/MeOH mixture (1 : 1, 1 mL each) at room temperature under argon and 410 nm irradiation for 0.5–6 h; conversions and product yields were determined by 1H NMR analysis from the crude product using chlorodiphenylmethane as an internal standard.
