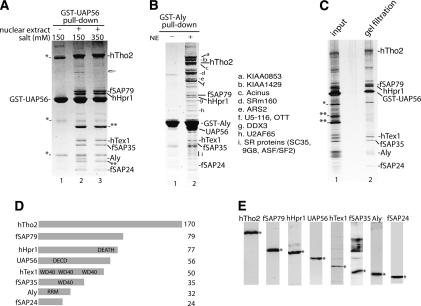
Figure 1.
Characterization of the human TREX complex. (A) GST-UAP56 pull-down in the absence (lane 1) or presence (lanes 2,3) of RNase-treated HeLa nuclear extract. The GST-UAP56 pull-down was washed with 150 mM salt (lane 2) or 350 mM salt (lane 3). The indicated bands were identified by mass spectrometry. (*) GST-UAP56 dimer and breakdown products; (**) GST-binding protein in HeLa nuclear extract; the fish icon indicates ARS2, a protein associated with the hTREX complex at low salt and substoichiometrically. (B) GST-Aly pull-down in the absence (lane 1) or presence (lane 2) of RNase-treated HeLa nuclear extracts. a–i are Aly-associated proteins not present in the hTREX complex. The identity of these proteins is shown to the right of B. Abundant proteins such as ribosomal proteins are not indicated. (C) The GST-UAP56 pull-down was eluted with glutathione, run on a Sephacryl S-500 gel filtration column, and fractions run on an SDS gel followed by silver staining. (Lane 1) Input. (Lane 2) Gel filtration fraction. (D) Schematics of hTREX complex proteins. (DEATH) Death domain; (DECD) DECD RNA helicase motif; (WD40) WD 40 repeat; (RRM) RNA recognition motif. (E) Western analysis of nuclear extract with the indicated antibody. The specific protein is designated with an asterisk.