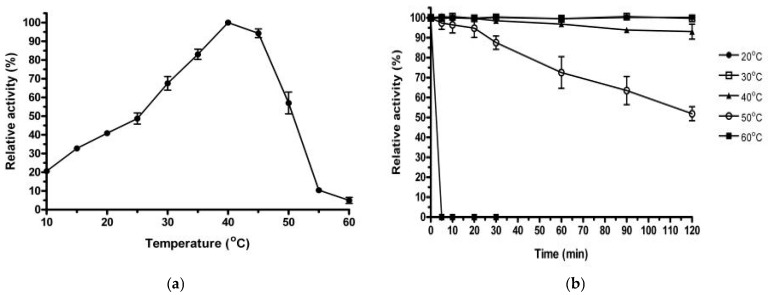
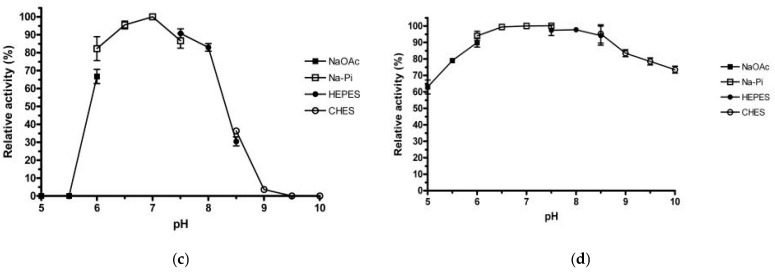
Figure 3.
Biochemical characterization of rManEM6. (a) The enzyme activity of rManEM6 (0.2 μg) was measured at various temperatures in 50 mM sodium phosphate (pH 7.0) for 10 min, exhibiting >50% hydrolytic activity in the range of 25–50 °C with an optimum at 40 °C. (b) The thermostability of rManEM6 was examined at 40 °C for 10 min after 4 h pre-incubation at the given temperature. (c) pH inhibition was monitored in the following 50 mM buffers: sodium acetate buffer (NaOAc, pH 5.0–6.0), sodium phosphate buffer (Na-Pi, pH 6.0–7.5), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (HEPES, pH 7.5–8.5), and 2-(cyclohexylamino) ethanesulfonic acid buffer (CHES, pH 8.5–10.0). (d) To verify pH stability, the enzyme was pre-incubated in 50 mM buffer with different pH values at 4 °C for 15 h, and residual activity was examined. Each β-mannanase assay was carried out by measuring the amount of mannose released from locust bean gum (0.5% [w/v]) under standard assay conditions, as described in the Materials and Methods section. Error bars represent SEM from triplicate results (p ≤ 0.01).